Modified Fluoroquinolones as Antimicrobial Compounds Targeting Chlamydia trachomatis
Abstract
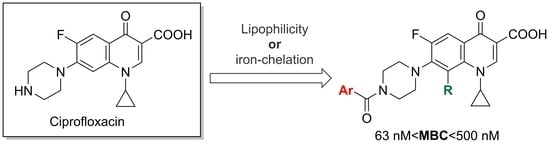
:1. Introduction
2. Results and Discussion
2.1. Organic Synthesis
2.2. Iron-Chelating Properties
2.3. Antibacterial Activity
3. Materials and Methods
3.1. Organic Synthesis
3.1.1. Materials and Methods
3.1.2. Experimental Procedures
3.2. Metal Chelation
3.2.1. Stock Solutions
3.2.2. Spectrophotometric Measurements
3.3. Antibacterial Activity
3.3.1. C. trachomatis
- Cell culture and Chlamydia propagation
- Cell viability assay
3.3.2. N. gonorrhoeae
3.3.3. Susceptibility Testing for ESKAPE Pathogens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, L.; Sun, Y.; Han, M.; Wang, B.; Xiao, F.; Zhou, Y.; Gao, Y.; Fitzpatrick, T.; Yuan, T.; Li, P.; et al. Incidence Trends of Five Common Sexually Transmitted Infections Excluding HIV From 1990 to 2019 at the Global, Regional, and National Levels: Results from the Global Burden of Disease Study 2019. Front. Med. 2022, 9, 851635. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef]
- Task Force for Global Health. Home | International Trachoma Initiative Accessed. Available online: https://www.trachoma.org (accessed on 24 April 2022).
- World Health Organization. WHO Guidelines for the Treatment of Chlamydia trachomatis. Available online: https://www.ncbi.nlm.nih.gov (accessed on 17 February 2022).
- Paradkar, P.N.; De Domenico, I.; Durchfort, N.; Zohn, I.; Kaplan, J.; Ward, D.M. Iron Depletion Limits Intracellular Bacterial Growth in Macrophages. Blood 2008, 112, 866–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raulston, J.E. Response of Chlamydia trachomatis Serovar E to Iron Restriction in Vitro and Evidence for Iron-Regulated Chlamydial Proteins. Infect. Immun. 1997, 65, 4539–4547. [Google Scholar] [CrossRef] [Green Version]
- Dill, B.D.; Dessus-Babus, S.; Raulston, J.E. Identification of Iron-Responsive Proteins Expressed by Chlamydia trachomatis Reticulate Bodies during Intracellular Growth. Microbiology 2009, 155, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.D.; Sal, M.S.; Schell, M.; Whittimore, J.D.; Raulston, J.E. Chlamydia trachomatis YtgA Is an Iron-Binding Periplasmic Protein Induced by Iron Restriction. Microbiology 2009, 155, 2884–2894. [Google Scholar] [CrossRef] [Green Version]
- Slepenkin, A.; Enquist, P.-A.; Hägglund, U.; de la Maza, L.M.; Elofsson, M.; Peterson, E.M. Reversal of the Antichlamydial Activity of Putative Type III Secretion Inhibitors by Iron. Infect. Immun. 2007, 75, 3478–3489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saka, H.A.; Thompson, J.W.; Chen, Y.-S.; Kumar, Y.; Dubois, L.G.; Moseley, M.A.; Valdivia, R.H. Quantitative Proteomics Reveals Metabolic and Pathogenic Properties of Chlamydia trachomatis Developmental Forms: Quantitative Proteomics Analysis of C. Trachomatis Developmental Forms. Mol. Microbiol. 2011, 82, 1185–1203. [Google Scholar] [CrossRef] [Green Version]
- Abdelsayed, S.; Ha Duong, N.T.; Hai, J.; Hémadi, M.; El Hage Chahine, J.M.; Verbeke, P.; Serradji, N. Design and Synthesis of 3-Isoxazolidone Derivatives as New Chlamydia trachomatis Inhibitors. Bioorganic Med. Chem. Lett. 2014, 24, 3854–3860. [Google Scholar] [CrossRef]
- Enquist, P.-A.; Gylfe, Å.; Hägglund, U.; Lindström, P.; Norberg-Scherman, H.; Sundin, C.; Elofsson, M. Derivatives of 8-Hydroxyquinoline—Antibacterial Agents That Target Intra- and Extracellular Gram-Negative Pathogens. Bioorganic Med. Chem. Lett. 2012, 22, 3550–3553. [Google Scholar] [CrossRef]
- Vu, T.H.; Ha-Duong, N.-T.; Aubry, A.; Capton, E.; Fechter, P.; Plésiat, P.; Verbeke, P.; Serradji, N. In Vitro Activities of a New Fluoroquinolone Derivative Highly Active against Chlamydia trachomatis. Bioorganic Chem. 2019, 83, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Chaudry, A.E.; Klausner, J.D. A Narrative Review of Clinical Treatment Outcomes of Neisseria gonorrhoeae Infection with Ciprofloxacin by Minimum Inhibitory Concentration and Anatomic Site. Sex. Transm. Dis. 2021, 48, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Da Costa-Lourenço, A.P.R.; Barros dos Santos, K.T.; Moreira, B.M.; Fracalanzza, S.E.L.; Bonelli, R.R. Antimicrobial Resistance in Neisseria gonorrhoeae: History, Molecular Mechanisms and Epidemiological Aspects of an Emerging Global Threat. Braz. J. Microbiol. 2017, 48, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Guruswamy, B.; Arul, R. Synthesis, Characterization, and Antimicrobial Activities of Novel N-Substituted β-Hydroxy Amines and β-Hydroxy Ethers That Contained 8-Methoxy Fluoroquinolones: Synthesis, Characterization, and Antimicrobial Activities of Novel N-Substituted β-Hydroxy Amines and β-Hydroxy Ethers That Contained 8-Methoxy Fluoro. J. Heterocycl. Chem. 2016, 53, 284–293. [Google Scholar] [CrossRef]
- Noël, S.; Gasser, V.; Pesset, B.; Hoegy, F.; Rognan, D.; Schalk, I.J.; Mislin, G.L.A. Synthesis and Biological Properties of Conjugates between Fluoroquinolones and a N3′′-Functionalized Pyochelin. Org. Biomol. Chem. 2011, 9, 8288. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Qu, L.; Wang, X.; Xu, T.; Xiao, X.; Ding, M.; Deng, L.; Guo, Y. Design, Synthesis, and Antibacterial Activity of Novel 8-Methoxyquinoline-2-Carboxamide Compounds Containing 1,3,4-Thiadiazole Moiety. Z. Nat. C 2018, 73, 117–122. [Google Scholar] [CrossRef]
- Terazzi, E.; Guénée, L.; Bocquet, B.; Lemonnier, J.-F.; Favera, N.D.; Piguet, C. A Simple Chemical Tuning of the Effective Concentration: Selection of Single-, Double-, and Triple-Stranded Binuclear Lanthanide Helicates. Chem. Eur. J. 2009, 15, 12719–12732. [Google Scholar] [CrossRef]
- Jisha, B.; Resmi, M.R.; Maya, R.J.; Varma, R.L. Colorimetric Detection of Al(III) Ions Based on Triethylene Glycol Appended 8-Propyloxy Quinoline Ester. Tetrahedron Lett. 2013, 54, 4232–4236. [Google Scholar] [CrossRef]
- Huyen, V.T.; Serradji, N.; Seydou, M.; Brémond, É.; Ha-Duong, N.-T. Electronic Spectroscopic Characterization of the Formation of Iron(III) Metal Complexes: The 8-HydroxyQuinoline as Ligand Case Study. J. Inorg. Biochem. 2020, 203, 110864. [Google Scholar] [CrossRef]
- Wang, R.; Lu, Y.; Wang, S. Comparative Evaluation of 11 Scoring Functions for Molecular Docking. J. Med. Chem. 2003, 46, 2287–2303. [Google Scholar] [CrossRef]
- Yang, X.; Cai, P.; Liu, Q.; Wu, J.; Yin, Y.; Wang, X.; Kong, L. Novel 8-Hydroxyquinoline Derivatives Targeting β-Amyloid Aggregation, Metal Chelation and Oxidative Stress against Alzheimer’s Disease. Bioorganic Med. Chem. 2018, 26, 3191–3201. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.P.; Gogliotti, R.D.; Domagala, J.M.; Gracheck, S.J.; Huband, M.D.; Sesnie, J.A.; Cohen, M.A.; Shapiro, M.A. The Synthesis, Structure-Activity, and Structure-Side Effect Relationships of a Series of 8-Alkoxy- and 5-Amino-8-Alkoxyquinolone Antibacterial Agents. J. Med. Chem. 1995, 38, 4478–4487. [Google Scholar] [CrossRef] [PubMed]
- Smythe, M.A.; Rybak, M.J. Ofloxacin: A Review. DICP Ann. Pharmacother. 1989, 23, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Yasuda, M.; Wada, K.; Matsumoto, M.; Hayami, H.; Kobayashi, K.; Miyazaki, J.; Kiyota, H.; Matsumoto, T.; Yotsuyanagi, H.; et al. Nationwide Surveillance of the Antimicrobial Susceptibility of Chlamydia trachomatis from Male Urethritis in Japan: Comparison with the First Surveillance Report. J. Infect. Chemother. 2022, 28, 1–5. [Google Scholar] [CrossRef]
- Bébéar, C.M.; de Barbeyrac, B.; Pereyre, S.; Renaudin, H.; Clerc, M.; Bébéar, C. Activity of Moxifloxacin against the Urogenital Mycoplasmas Ureaplasma Spp., Mycoplasma Hominis and Mycoplasma Genitalium and Chlamydia trachomatis. Clin. Microbiol. Infect. 2008, 14, 801–805. [Google Scholar] [CrossRef] [Green Version]
- Mojica, S.A.; Eriksson, A.U.; Davis, R.A.; Bahnan, W.; Elofsson, M.; Gylfe, Å. Red Fluorescent Chlamydia trachomatis Applied to Live Cell Imaging and Screening for Antibacterial Agents. Front. Microbiol. 2018, 9, 3151. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The Influence of Lipophilicity in Drug Discovery and Design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- Jonsson, A.; Foester, S.; Golparian, D.; Hamasuna, R.; Jacobsson, S.; Lindberg, M.; Jensen, J.S.; Ohnishi, M.; Unemo, M. In Vitro Activity and Time-kill Curve Analysis of Sitafloxacin against a Global Panel of Antimicrobial-resistant and Multidrug-resistant Neisseria gonorrhoeae Isolates. Acta Pathol. Microbiol. Immunol. Scand. 2018, 126, 29–37. [Google Scholar] [CrossRef]
- Cornelissen, C.N. Subversion of Nutritional Immunity by the Pathogenic Neisseriae. Pathog. Dis. 2018, 76, ftx112. [Google Scholar] [CrossRef] [Green Version]
- El Hage Chahine, J.-M.; Fain, D. The Mechanism of Iron Release from Transferrin. Slow-Proton-Transfer-Induced Loss of Nitrilotriacetatoiron(III) Complex in Acidic Media. Eur. J. Biochem. 1994, 223, 581–587. [Google Scholar] [CrossRef]
- Binstead, R.A.; Zuberbühler, A.D.; Jung, B. SPECFIT Global Analysis System, v3.04.34; Spectrum Software Associates: Chapel Hill, NC, USA, 2003. [Google Scholar]
- Caldwell, H.D.; Kromhout, J.; Schachter, J. Purification and Partial Characterization of the Major Outer Membrane Protein of Chlamydia trachomatis. Infect. Immun. 1981, 31, 1161–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwaha, S.; Uvell, H.; Salin, O.; Lindgren, A.E.G.; Silver, J.; Elofsson, M.; Gylfe, Å. N-Acylated Derivatives of Sulfamethoxazole and Sulfafurazole Inhibit Intracellular Growth of Chlamydia trachomatis. Antimicrob. Agents Chemother. 2014, 58, 2968–2971. [Google Scholar] [CrossRef] [Green Version]
- Good, J.A.D.; Silver, J.; Nunez-Otero, C.; Bahnan, W.; Krishnan, K.S.; Salin, O.; Engström, P.; Svensson, R.; Artursson, P.; Gylfe, Å.; et al. Thiazolino 2-Pyridone Amide Inhibitors of Chlamydia trachomatis Infectivity. J. Med. Chem. 2016, 59, 2094–2108. [Google Scholar] [CrossRef] [PubMed]
- Stokes, W.S.; Casati, S.; Strickland, J.; Paris, M. Neutral Red Uptake Cytotoxicity Tests for Estimating Starting Doses for Acute Oral Toxicity Tests. Curr. Protoc. Toxicol. 2008, 36, 20.4.1–20.4.20. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Fasth, O.; Fredlund, H.; Limnios, A.; Tapsall, J. Phenotypic and Genetic Characterization of the 2008 WHO Neisseria gonorrhoeae Reference Strain Panel Intended for Global Quality Assurance and Quality Control of Gonococcal Antimicrobial Resistance Surveillance for Public Health Purposes. J. Antimicrob. Chemother. 2009, 63, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, C.P.; Stock, F.; Gill, V.J. Improved Enrichment Broth for Cultivation of Fastidious Organisms. J. Clin. Microbiol. 1994, 32, 1825–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foerster, S.; Gustafsson, T.N.; Brochado, A.R.; Desilvestro, V.; Typas, A.; Unemo, M. The First Wide-Scale Drug Repurposing Screen Using the Prestwick Chemical Library (1200 Bioactive Molecules) against Neisseria gonorrhoeae Identifies High in Vitro Activity of Auranofin and Many Additional Drugs. Acta Pathol. Mircobiologica Immunol. Scand. 2020, 128, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Anquetin, G.; Greiner, J.; Mahmoudi, N.; Santillana-Hayat, M.; Gozalbes, R.; Farhati, K.; Derouin, F.; Aubry, A.; Cambau, E.; Vierling, P. Design, Synthesis and Activity against Toxoplasma Gondii, Plasmodium Spp., and Mycobacterium Tuberculosis of New 6-Fluoroquinolones. Eur. J. Med. Chem. 2006, 41, 1478–1493. [Google Scholar] [CrossRef]








| Compound | LMCT Bands (nm) |
|---|---|
 1 | 430 |
 Ciprofloxacin | 430–450 |
 6 | 435 and 570 |
 HQ | 462 and 560 |
| C. trachomatis | N. gonorrhoeae WHO-P | N. gonorrhoeae WHO-G | |
|---|---|---|---|
| Compound | MIC (µM) | MIC/MBC (µM) | MIC/MBC (µM) |
| Ciprofloxacin | 0.5 | 0.008/0.016 | 0.25/1 |
| 1 | 0.25 | 0.125/0.125 | 2/4 |
| 5a | 0.25 | 0.25/1 | 4/8 |
| 5b | 0.25 | 2/2 | >12/>12 |
| 6 | 0.125 | 0.5/2 | >12/>12 |
| 5d | 0.5 | 0.5/2 | 16/>25 |
| 14 | 0.25 | 0.031/0.031 | 1/4 |
| 5c | >1.25 | >4/>4 | >4/>4 |
| 7 | 0.5 | 0.25/0.5 | 2/4 |
| 8 | 2.5 | 0.125/0.25 | 2/4 |
| 9 | 10 | 0.5/1 | 8/16 |
| 10 | 2.5 | 0.125/0.125 | 2/4 |
| 11 | 1 | 0.125/0.25 | 2/4 |
| 12 | 1 | 0.5/0.5 | 4/8 |
| 15 | 0.125 | 0.016/0.031 | 1/1 |
| 16 | >2.5 | 0.125/0.25 | 2/4 |
| C. trachomatis Progeny | C. trachomatis Progeny | cLog P a | |
|---|---|---|---|
| Compound | EC50 (95% CI) (nM) | MBC (nM) | |
| 1 | 32 (30–34) | 250 | 3.09 |
| 5a | 47 (44–51) | 250 | 3.24 |
| 5b | 46 (43–51) | 125 | 4.24 |
| 5d | 130 (120–142) | 500 | 5 |
| 6 | 13 (12–14) | 63 | - |
| 14 | 59 (56–62) | 500 | - |
| 15 | 28 (26–30) | 125 | - |
| Compounds | ≥95% Inhibition (μM) | With 200 µM Fe(III) Citrate ≥95% Inhibition (μM) | Compound Activity with Fe(III) Citrate |
|---|---|---|---|
| 1 | 0.25 | 0.25 | Similar |
| Ciprofloxacin | 0.5 | 0.5 | Similar |
| 14 | 0.25 | 0.5 | Similar |
| 5a | 0.25 | 0.25 | Similar |
| 6 | 0.125 | 1 | Decreased |
| Compound | Concentration (µM) | Cell Viability in HeLa 229 Cells |
|---|---|---|
| % of DMSO Control ± SD | ||
| Ciprofloxacin | 10 | 98.9 ± 4.5 |
| 1 | 10 | 113.2 ± 2.9 |
| 5a | 10 | 109.3 ± 1.2 |
| 5b | 10 | 107.8 ± 3.6 |
| 5c | 10 | n.a. |
| 5d | 10 | 105.1 ± 3.9 |
| 6 | 10 | 80.2 ± 3.1 |
| 7 | 10 | 79.0 ± 3.3 |
| 8 | 10 | 95.9 ± 6.8 |
| 9 | 10 | n.a. |
| 10 | 10 | 99.9 ± 3.5 |
| 11 | 10 | 92.0 ± 6.6 |
| 12 | 10 | 42.6 ± 3.1 |
| 14 | 10 | 86.9 ± 4.8 |
| 15 * | 1.25 | 102.6 ± 8.3 |
| 16 | 10 | n.a. |
| Compounds Tested | MIC (μM) | ||||
|---|---|---|---|---|---|
| Staphylococcus aureus | Enterococcus faecalis UCN41 | Enterococcus faecium | |||
| ATCC 25923 | ATCC 700699 | BM4147 | ATCC 19434T | ||
| Ciprofloxacin | 0.78 | 100 | 0.20 | 12.5 | 12.5 |
| 1 | 0.78 | >100 | 12.5 | >100 | >100 |
| 5a | 0.20 | >100 | 1.56 | 100 | >100 |
| 5b | 25 | >100 | 100 | >100 | >100 |
| 6 | 1.56 | >100 | 12.5 | >100 | >100 |
| 5d | >100 | >100 | >100 | >100 | >100 |
| Compounds tested | MIC (μM) | |||
|---|---|---|---|---|
| E. coli | Klebsiella pneumoniae | Pseudomonas aeruginosa | Acinetobacter baumannii | |
| ATCC 25922 | ATCC 700603 | PAO1 | CIP7010 | |
| Ciprofloxacin | ≤0.10 | 0.39 | ≤0.10 | 0.20 |
| 1 | 0.20 | 12.50 | 3.12 | 3.12 |
| 5a | 0.78 | 100 | 25 | 25 |
| 5b | 12.50 | >100 | >100 | >100 |
| 6 | 6.25 | >100 | >100 | 25 |
| 5d | 6.25 | >100 | >100 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, T.H.; Adhel, E.; Vielfort, K.; Ha Duong, N.-T.; Anquetin, G.; Jeannot, K.; Verbeke, P.; Hjalmar, S.; Gylfe, Å.; Serradji, N. Modified Fluoroquinolones as Antimicrobial Compounds Targeting Chlamydia trachomatis. Int. J. Mol. Sci. 2022, 23, 6741. https://doi.org/10.3390/ijms23126741
Vu TH, Adhel E, Vielfort K, Ha Duong N-T, Anquetin G, Jeannot K, Verbeke P, Hjalmar S, Gylfe Å, Serradji N. Modified Fluoroquinolones as Antimicrobial Compounds Targeting Chlamydia trachomatis. International Journal of Molecular Sciences. 2022; 23(12):6741. https://doi.org/10.3390/ijms23126741
Chicago/Turabian StyleVu, Thi Huyen, Erika Adhel, Katarina Vielfort, Ngûyet-Thanh Ha Duong, Guillaume Anquetin, Katy Jeannot, Philippe Verbeke, Sofia Hjalmar, Åsa Gylfe, and Nawal Serradji. 2022. "Modified Fluoroquinolones as Antimicrobial Compounds Targeting Chlamydia trachomatis" International Journal of Molecular Sciences 23, no. 12: 6741. https://doi.org/10.3390/ijms23126741