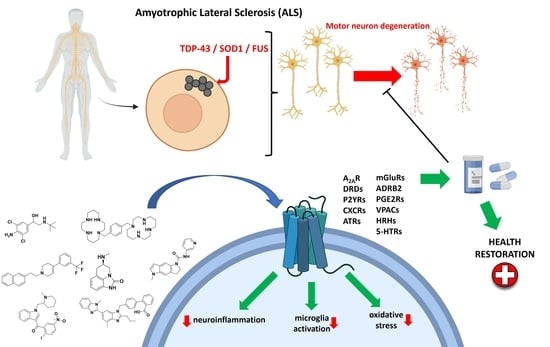
The Multifaceted Role of GPCRs in Amyotrophic Lateral Sclerosis: A New Therapeutic Perspective?
Abstract
:1. Introduction
2. GPCRs Involved in ALS
2.1. Puringergic Receptors P2Y and Adenosine Receptor A2AAR
2.2. Chemokine Receptors
2.3. Angiotensin II Receptors (ATRs)
2.4. Dopamine Receptors
2.5. Serotonin (5-HT) Receptors
2.6. GPR17 Receptor
2.7. Adrenergic Receptor β2
2.8. Histamine Receptors
2.9. Cannabinoid Receptors
2.10. Prostaglandin E2 Receptor (PGE2R)
2.11. Vasoactive Intestinal Peptide Receptors
2.12. Metabotropic Glutamate Receptors (mGluRs)
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adams, J.; Lee, M.; Peng, W. Critical Review of Complementary and Alternative Medicine Use in Amyotrophic Lateral Sclerosis: Prevalence and Users’ Profile, Decision-Making, Information Seeking, and Disclosure in the Face of a Lack of Efficacy. Neurodegener. Dis. 2018, 18, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Heckman, C.; Enoka, R.M. Physiology of the motor neuron and the motor unit. In Handbook of Clinical Neurophysiology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 119–147. [Google Scholar] [CrossRef]
- Wijesekera, L.C.; Leigh, P.N. Amyotrophic lateral sclerosis. Orphanet J. Rare Dis. 2009, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardiman, O.; Al-Chalabi, A.; Chiò, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortí, J.D.L.R.; Armero, J.; Sanchis-Sanchis, C.; Sancho-Castillo, S.; Salazar, A.; Caplliure-Llopis, J.; Navarro-Illana, E.; Barrios, C.; Escribá-Alepuz, J.; Benlloch, M. Muscle Function Differences between Patients with Bulbar and Spinal Onset Amyotrophic Lateral Sclerosis. Does It Depend on Peripheral Glucose? J. Clin. Med. 2021, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke. Amyotrophic Lateral Sclerosis (ALS) Fact Sheet. Available online: www.ninds.nih.gov (accessed on 22 March 2022).
- Saitoh, Y.; Takahashi, Y. Riluzole for the treatment of amyotrophic lateral sclerosis. Neurodegener. Dis. Manag. 2020, 10, 343–355. [Google Scholar] [CrossRef]
- Breiner, A.; Zinman, L.; Bourque, P.R. Edaravone for amyotrophic lateral sclerosis: Barriers to access and lifeboat ethics. Can. Med. Assoc. J. 2020, 192, E319–E320. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, M.K. Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med. Res. Rev. 2018, 39, 733–748. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Boddy, S.L.; Giovannelli, I.; Sassani, M.; Cooper-Knock, J.; Snyder, M.P.; Segal, E.; Elinav, E.; Barker, L.A.; Shaw, P.J.; McDermott, C.J. The gut microbiome: A key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 2021, 19, 13. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Jo, M.; Lee, S.; Jeon, Y.-M.; Kim, S.; Kwon, Y.; Kim, H.-J. The role of TDP-43 propagation in neurodegenerative diseases: Integrating insights from clinical and experimental studies. Exp. Mol. Med. 2020, 52, 1652–1662. [Google Scholar] [CrossRef]
- Suk, T.; Rousseaux, M.W.C. The role of TDP-43 mislocalization in amyotrophic lateral sclerosis. Mol. Neurodegener. 2020, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Berdyński, M.; Miszta, P.; Safranow, K.; Andersen, P.M.; Morita, M.; Filipek, S.; Żekanowski, C.; Kuźma-Kozakiewicz, M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 2022, 12, 103. [Google Scholar] [CrossRef]
- Ishigaki, S.; Sobue, G. Importance of Functional Loss of FUS in FTLD/ALS. Front. Mol. Biosci. 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Eck, R.J.; Kraemer, B.C.; Liachko, N.F. Regulation of TDP-43 phosphorylation in aging and disease. GeroScience 2021, 43, 1605–1614. [Google Scholar] [CrossRef]
- Guo, W.; Vandoorne, T.; Steyaert, J.; Staats, K.A.; Van Den Bosch, L. The multifaceted role of kinases in amyotrophic lateral sclerosis: Genetic, pathological and therapeutic implications. Brain 2020, 143, 1651–1673. [Google Scholar] [CrossRef]
- Palomo, V.; Nozal, V.; Rojas-Prats, E.; Gil, C.; Martinez, A. Protein kinase inhibitors for amyotrophic lateral sclerosis therapy. J. Cereb. Blood Flow Metab. 2020, 178, 1316–1335. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Mitchell, J.D.; Moore, D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. 2012, 65, CD001447. [Google Scholar] [CrossRef]
- Moro, S.; Bissaro, M. Rethinking to riluzole mechanism of action: The molecular link among protein kinase CK1δ activity, TDP-43 phosphorylation, and amyotrophic lateral sclerosis pharmacological treatment. Neural Regen. Res. 2019, 14, 2083–2085. [Google Scholar] [CrossRef]
- Corcia, P.; Beltran, S.; Bakkouche, S.; Couratier, P. Therapeutic news in ALS. Rev. Neurol. 2021, 177, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Springer Nature. AdisInsight—Small-Molecules in Clinical Trials for ALS. Available online: https://adisinsight.springer.com/search (accessed on 31 March 2022).
- Chen, H.; Kankel, M.W.; Su, S.C.; Han, S.W.S.; Ofengeim, D. Exploring the genetics and non-cell autonomous mechanisms underlying ALS/FTLD. Cell Death Differ. 2018, 25, 648–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Insel, P.A.; Wilderman, A.; Zambon, A.C.; Snead, A.N.; Murray, F.; Aroonsakool, N.; McDonald, D.S.; Zhou, S.; McCann, T.; Zhang, L.; et al. G Protein–Coupled Receptor (GPCR) Expression in Native Cells: “Novel” endoGPCRs as Physiologic Regulators and Therapeutic Targets. Mol. Pharmacol. 2015, 88, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gacasan, S.B.; Baker, D.L.; Parrill, A.L. G protein-coupled receptors: The evolution of structural insight. AIMS Biophys. 2017, 4, 491–527. [Google Scholar] [CrossRef]
- Lee, Y.; Basith, S.; Choi, S. Recent Advances in Structure-Based Drug Design Targeting Class A G Protein-Coupled Receptors Utilizing Crystal Structures and Computational Simulations. J. Med. Chem. 2017, 61, 1–46. [Google Scholar] [CrossRef]
- Heng, B.C.; Aubel, D.; Fussenegger, M. An overview of the diverse roles of G-protein coupled receptors (GPCRs) in the pathophysiology of various human diseases. Biotechnol. Adv. 2013, 31, 1676–1694. [Google Scholar] [CrossRef]
- Gomes, C.; Kaster, M.; Tome, A.; Agostinho, P.; Cunha, R.A. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim. Biophys. Acta 2011, 1808, 1380–1399. [Google Scholar] [CrossRef] [Green Version]
- Vincenzi, F.; Corciulo, C.; Targa, M.; Casetta, I.; Gentile, M.; Granieri, E.; Borea, P.A.; Popoli, P.; Varani, K. A2Aadenosine receptors are up-regulated in lymphocytes from amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 406–413. [Google Scholar] [CrossRef]
- Yoshida, Y.; Une, F.; Utatsu, Y.; Nomoto, M.; Furukawa, Y.; Maruyama, Y.; Machigashira, N.; Matsuzaki, T.; Osame, M. Adenosine and Neopterin Levels in Cerebrospinal Fluid of Patients with Neurological Disorders. Intern. Med. 1999, 38, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Potenza, R.L.; Armida, M.; Ferrante, A.; Pèzzola, A.; Matteucci, A.; Puopolo, M.; Popoli, P. Effects of chronic caffeine intake in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. Res. 2013, 91, 585–592. [Google Scholar] [CrossRef]
- Mori, A.; Cross, B.; Uchida, S.; Walker, J.K.; Ristuccia, R. How Are Adenosine and Adenosine A2A Receptors Involved in the Pathophysiology of Amyotrophic Lateral Sclerosis? Biomedicines 2021, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.K.; Higashimori, H.; Tolman, M.; Yang, Y. Suppression of adenosine 2a receptor (A2aR)-mediated adenosine signaling improves disease phenotypes in a mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2015, 267, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojsilovic-Petrovic, J.; Jeong, G.-B.; Crocker, A.; Arneja, A.; David, S.; Russell, D.; Kalb, R.G. Protecting Motor Neurons from Toxic Insult by Antagonism of Adenosine A2a and Trk Receptors. J. Neurosci. 2006, 26, 9250–9263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Ju, T.; Chen, H.-M.; Jang, Y.-S.; Lee, L.-M.; Lai, H.-L.; Tai, H.-C.; Fang, J.-M.; Lin, Y.-L.; Tu, P.-H.; et al. Activation of AMP-activated protein kinase α1 mediates mislocalization of TDP-43 in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2014, 24, 787–801. [Google Scholar] [CrossRef]
- Morillas, A.G.; Besson, V.; Lerouet, D. Microglia and Neuroinflammation: What Place for P2RY12? Int. J. Mol. Sci. 2021, 22, 1636. [Google Scholar] [CrossRef]
- Amadio, S.; Parisi, C.; Montilli, C.; Carrubba, A.S.; Apolloni, S.; Volonté, C. P2Y12 Receptor on the Verge of a Neuroinflammatory Breakdown. Mediat. Inflamm. 2014, 2014, 975849. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; Von Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. J. Cereb. Blood Flow Metab. 2020, 177, 2413–2433. [Google Scholar] [CrossRef]
- D’Ambrosi, N.; Finocchi, P.; Apolloni, S.; Cozzolino, M.; Ferri, A.; Padovano, V.; Pietrini, G.; Carrì, M.T.; Volonté, C. The Proinflammatory Action of Microglial P2 Receptors Is Enhanced in SOD1 Models for Amyotrophic Lateral Sclerosis. J. Immunol. 2009, 183, 4648–4656. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Yamanaka, H.; Fukuoka, T.; Dai, Y.; Obata, K.; Noguchi, K. P2Y12 Receptor Upregulation in Activated Microglia Is a Gateway of p38 Signaling and Neuropathic Pain. J. Neurosci. 2008, 28, 2892–2902. [Google Scholar] [CrossRef]
- Moore, C.S.; Ase, A.R.; Kinsara, A.; Rao, V.T.; Michell-Robinson, M.; Leong, S.Y.; Butovsky, O.; Ludwin, S.K.; Séguéla, P.; Bar-Or, A.; et al. P2Y12 expression and function in alternatively activated human microglia. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e80. [Google Scholar] [CrossRef] [Green Version]
- La Cognata, V.; Golini, E.; Iemmolo, R.; Balletta, S.; Morello, G.; De Rosa, C.; Villari, A.; Marinelli, S.; Vacca, V.; Bonaventura, G.; et al. CXCR2 increases in ALS cortical neurons and its inhibition prevents motor neuron degeneration in vitro and improves neuromuscular function in SOD1G93A mice. Neurobiol. Dis. 2021, 160, 105538. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Nikitin, I.; Ezra, A.; Barbiro, B.; Rabinovich-Toidman, P.; Solomon, B. Chronic administration of AMD3100 increases survival and alleviates pathology in SOD1G93A mice model of ALS. J. Neuroinflamm. 2016, 13, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, E.; Karpf, L.; Bohl, D. Neuroinflammation in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia and the Interest of Induced Pluripotent Stem Cells to Study Immune Cells Interactions with Neurons. Front. Mol. Neurosci. 2021, 14, 767041. [Google Scholar] [CrossRef] [PubMed]
- Perner, C.; Perner, F.; Stubendorff, B.; Förster, M.; Witte, O.W.; Heidel, F.H.; Prell, T.; Grosskreutz, J. Dysregulation of chemokine receptor expression and function in leukocytes from ALS patients. J. Neuroinflamm. 2018, 15, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawajiri, M.; Mogi, M.; Higaki, N.; Tateishi, T.; Ohyagi, Y.; Horiuchi, M.; Miki, T.; Kira, J.-I. Reduced angiotensin II levels in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neurol. Scand. 2009, 119, 341–344. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ichikawa, Y.; Igarashi, O.; Kinoshita, M.; Ikeda, K. Trophic effect of Olmesartan, a novel AT1R antagonist, on spinal motor neuronsin vitroandin vivo. Neurol. Res. 2002, 24, 468–472. [Google Scholar] [CrossRef]
- Mammana, S.; Fagone, P.; Cavalli, E.; Basile, M.S.; Petralia, M.C.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The Role of Macrophages in Neuroinflammatory and Neurodegenerative Pathways of Alzheimer’s Disease, Amyotrophic Lateral Sclerosis, and Multiple Sclerosis: Pathogenetic Cellular Effectors and Potential Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 831. [Google Scholar] [CrossRef] [Green Version]
- Borasio, G.D.; Linke, R.; Schwarz, J.; Schlamp, V.; Abel, A.; Mozley, P.D.; Tatsch, K. Dopaminergic deficit in amyotrophic lateral sclerosis assessed with [I-123] IPT single photon emission computed tomography. J. Neurol. Neurosurg. Psychiatry 1998, 65, 263–265. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-Y.; Liu, Y.-J.; Lai, H.-L.; Chen, H.-M.; Kuo, H.-C.; Liao, Y.-P.; Chern, Y. The D2 Dopamine Receptor Interferes With the Protective Effect of the A2A Adenosine Receptor on TDP-43 Mislocalization in Experimental Models of Motor Neuron Degeneration. Front. Neurosci. 2018, 12, 187. [Google Scholar] [CrossRef]
- Huang, X.; Roet, K.C.; Zhang, L.; Brault, A.; Berg, A.P.; Jefferson, A.B.; Klug-McLeod, J.; Leach, K.L.; Vincent, F.; Yang, H.; et al. Human amyotrophic lateral sclerosis excitability phenotype screen: Target discovery and validation. Cell Rep. 2021, 35, 109224. [Google Scholar] [CrossRef]
- Fujimori, K.; Ishikawa, M.; Otomo, A.; Atsuta, N.; Nakamura, R.; Akiyama, T.; Hadano, S.; Aoki, M.; Saya, H.; Sobue, G.; et al. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat. Med. 2018, 24, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Dooley, M.; Markham, A. Pramipexole. Drugs Aging 1998, 12, 495–514. [Google Scholar] [CrossRef] [PubMed]
- Gribkoff, V.K.; Bozik, M.E. KNS-760704 [(6R)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine dihydrochloride monohydrate] for the Treatment of Amyotrophic Lateral Sclerosis. CNS Neurosci. Ther. 2008, 14, 215–226. [Google Scholar] [CrossRef]
- Kingwell, K. Dexpramipexole shows promise for ALS in phase II trial. Nat. Rev. Neurol. 2011, 8, 4. [Google Scholar] [CrossRef]
- Cudkowicz, M.E.; Berg, L.H.V.D.; Shefner, J.M.; Mitsumoto, H.; Mora, J.S.; Ludolph, A.; Hardiman, O.; Bozik, M.E.; Ingersoll, E.W.; Archibald, D.; et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): A randomised, double-blind, phase 3 trial. Lancet Neurol. 2013, 12, 1059–1067. [Google Scholar] [CrossRef]
- El Oussini, H.; Bayer, H.; Scekic-Zahirovic, J.; Vercruysse, P.; Sinniger, J.; Dirrig-Grosch, S.; Dieterlé, S.; Echaniz-Laguna, A.; Larmet, Y.; Müller, K.; et al. Serotonin 2B receptor slows disease progression and prevents degeneration of spinal cord mononuclear phagocytes in amyotrophic lateral sclerosis. Acta Neuropathol. 2016, 131, 465–480. [Google Scholar] [CrossRef] [Green Version]
- Dentel, C.; Palamiuc, L.; Henriques, A.; Lannes, B.; Spreux-Varoquaux, O.; Gutknecht, L.; René, F.; Echaniz-Laguna, A.; De Aguilar, J.-L.G.; Lesch, K.P.; et al. Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: A link to spasticity. Brain 2013, 136, 483–493. [Google Scholar] [CrossRef]
- Arnoux, A.; Ayme-Dietrich, E.; Dieterle, S.; Goy, M.-A.; Schann, S.; Frauli, M.; Monassier, L.; Dupuis, L. Evaluation of a 5-HT2B receptor agonist in a murine model of amyotrophic lateral sclerosis. Sci. Rep. 2021, 11, 23582. [Google Scholar] [CrossRef]
- Elangbam, C.S.; Job, L.E.; Zadrozny, L.M.; Barton, J.C.; Yoon, L.W.; Gates, L.D.; Slocum, N. 5-Hydroxytryptamine (5HT)-induced valvulopathy: Compositional valvular alterations are associated with 5HT2B receptor and 5HT transporter transcript changes in Sprague-Dawley rats. Exp. Toxicol. Pathol. 2008, 60, 253–262. [Google Scholar] [CrossRef]
- Lacomblez, L.; Bensimon, G.; Douillet, P.; Doppler, V.; Salachas, F.; Meininger, V. Xaliproden in amyotrophic lateral sclerosis: Early clinical trials. Amyotroph. Lateral Scler. 2004, 5, 99–106. [Google Scholar] [CrossRef]
- Lecca, D.; Raffaele, S.; Abbracchio, M.P.; Fumagalli, M. Regulation and signaling of the GPR17 receptor in oligodendroglial cells. Glia 2020, 68, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhao, C.; Zhang, X.; Huang, X.; Shi, W.; Fang, S.; Lu, Y.; Zhang, W.; Xia, Q.; Wei, E. The new P2Y-like receptor G protein-coupled receptor 17 mediates acute neuronal injury and late microgliosis after focal cerebral ischemia in rats. Neuroscience 2011, 202, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, E.; Bonifacino, T.; Raffaele, S.; Milanese, M.; Morgante, E.; Bonanno, G.; Abbracchio, M.P.; Fumagalli, M. Abnormal Upregulation of GPR17 Receptor Contributes to Oligodendrocyte Dysfunction in SOD1 G93A Mice. Int. J. Mol. Sci. 2020, 21, 2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merten, N.; Fischer, J.; Simon, K.; Zhang, L.; Schröder, R.; Peters, L.; Letombe, A.-G.; Hennen, S.; Schrage, R.; Bödefeld, T.; et al. Repurposing HAMI3379 to Block GPR17 and Promote Rodent and Human Oligodendrocyte Differentiation. Cell Chem. Biol. 2018, 25, 775–786.e5. [Google Scholar] [CrossRef] [Green Version]
- Raffaele, S.; Boccazzi, M.; Fumagalli, M. Oligodendrocyte Dysfunction in Amyotrophic Lateral Sclerosis: Mechanisms and Therapeutic Perspectives. Cells 2021, 10, 565. [Google Scholar] [CrossRef]
- Jin, S.; Wang, X.; Xiang, X.; Wu, Y.; Hu, J.; Li, Y.; Dong, Y.L.; Tan, Y.; Wu, X. Inhibition of GPR17 with cangrelor improves cognitive impairment and synaptic deficits induced by Aβ1–42 through Nrf2/HO-1 and NF-κB signaling pathway in mice. Int. Immunopharmacol. 2021, 101, 108335. [Google Scholar] [CrossRef]
- Marschallinger, J.; Schäffner, I.; Klein, B.; Gelfert, R.; Rivera, F.J.; Illes, S.; Grassner, L.; Janssen, M.; Rotheneichner, P.; Schmuckermair, C.; et al. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat. Commun. 2015, 6, 8466. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology 2016, 104, 4–17. [Google Scholar] [CrossRef]
- Bartus, R.T.; Bétourné, A.; Basile, A.; Peterson, B.L.; Glass, J.; Boulis, N.M. β 2 -Adrenoceptor agonists as novel, safe and potentially effective therapies for Amyotrophic lateral sclerosis (ALS). Neurobiol. Dis. 2016, 85, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.D.; Choi, H.; Huang, W.; Onario, R.C.; Frontera, W.R.; Snyder, E.Y.; Sabharwal, S. Therapeutic effects of clenbuterol in a murine model of amyotrophic lateral sclerosis. Neurosci. Lett. 2006, 397, 155–158. [Google Scholar] [CrossRef]
- Apolloni, S.; Fabbrizio, P.; Amadio, S.; Napoli, G.; Verdile, V.; Morello, G.; Iemmolo, R.; Aronica, E.; Cavallaro, S.; Volonté, C. Histamine Regulates the Inflammatory Profile of SOD1-G93A Microglia and the Histaminergic System Is Dysregulated in Amyotrophic Lateral Sclerosis. Front. Immunol. 2017, 8, 1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volonté, C.; Apolloni, S.; Sabatelli, M. Histamine beyond its effects on allergy: Potential therapeutic benefits for the treatment of Amyotrophic Lateral Sclerosis (ALS). Pharmacol. Ther. 2019, 202, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Zhang, Y.; Qu, C.; Zhou, X.; Zhang, S. Histamine Induces Microglia Activation and the Release of Proinflammatory Mediators in Rat Brain Via H1R or H4R. J. Neuroimmune Pharmacol. 2019, 15, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Apolloni, S.; Fabbrizio, P.; Parisi, C.; Amadio, S.; Volonté, C. Clemastine Confers Neuroprotection and Induces an Anti-Inflammatory Phenotype in SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2014, 53, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, E.; Giacoppo, S. Can cannabinoids be a potential therapeutic tool in amyotrophic lateral sclerosis? Neural Regen. Res. 2016, 11, 1896–1899. [Google Scholar] [CrossRef] [PubMed]
- Urbi, B.; Owusu, M.A.; Hughes, I.; Katz, M.; Broadley, S.; Sabet, A. Effects of cannabinoids in Amyotrophic Lateral Sclerosis (ALS) murine models: A systematic review and meta-analysis. J. Neurochem. 2018, 149, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, J.L.; Seely, K.A.; Reed, R.L.; Crow, J.P.; Prather, P.L. The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J. Neurochem. 2006, 101, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Moore, D.H.; Makriyannis, A.; Abood, M.E. AM1241, a cannabinoid CB2 receptor selective compound, delays disease progression in a mouse model of amyotrophic lateral sclerosis. Eur. J. Pharmacol. 2006, 542, 100–105. [Google Scholar] [CrossRef]
- Iłżecka, J. Prostaglandin E2 is increased in amyotrophic lateral sclerosis patients. Acta Neurol. Scand. 2003, 108, 125–129. [Google Scholar] [CrossRef]
- Kosuge, Y.; Nango, H.; Kasai, H.; Yanagi, T.; Mawatari, T.; Nishiyama, K.; Miyagishi, H.; Ishige, K.; Ito, Y. Generation of Cellular Reactive Oxygen Species by Activation of the EP2 Receptor Contributes to Prostaglandin E2-Induced Cytotoxicity in Motor Neuron-Like NSC-34 Cells. Oxidative Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Bilak, M.; Wu, L.; Wang, Q.; Haughey, N.; Conant, K.; St. Hillaire, C.; Andreasson, K. PGE2 receptors rescue motor neurons in a model of amyotrophic lateral sclerosis. Ann. Neurol. 2004, 56, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Staines, D.R. Are multiple sclerosis and amyotrophic lateral sclerosis autoimmune disorders of endogenous vasoactive neuropeptides? Med. Hypotheses 2008, 70, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Ikeda, K.; Ichikawa, Y.; Igarashi, O. Vasoactive intestinal peptide influences neurite outgrowth in cultured rat spinal cord neurons. Neurol. Res. 2001, 23, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Solés-Tarrés, I.; Cabezas-Llobet, N.; Vaudry, D.; Xifró, X. Protective Effects of Pituitary Adenylate Cyclase-Activating Polypeptide and Vasoactive Intestinal Peptide against Cognitive Decline in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 221. [Google Scholar] [CrossRef]
- Waschek, J. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. J. Cereb. Blood Flow Metab. 2013, 169, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Martínez, C.; Juarranz, Y.; Gutiérrez-Cañas, I.; Carrión, M.; Pérez-García, S.; Villanueva-Romero, R.; Castro, D.; Lamana, A.; Mellado, M.; González-Álvaro, I.; et al. A Clinical Approach for the Use of VIP Axis in Inflammatory and Autoimmune Diseases. Int. J. Mol. Sci. 2019, 21, 65. [Google Scholar] [CrossRef] [Green Version]
- Anneser, J.M.H.; Chahli, C.; Ince, P.G.; Borasio, G.D.; Shaw, P. Glial Proliferation and Metabotropic Glutamate Receptor Expression in Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2004, 63, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Milanese, M.; Giribaldi, F.; Melone, M.; Bonifacino, T.; Musante, I.; Carminati, E.; Rossi, P.I.; Vergani, L.; Voci, A.; Conti, F.; et al. Knocking down metabotropic glutamate receptor 1 improves survival and disease progression in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2014, 64, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Rossi, D.; Brambilla, L.; Valori, C.F.; Roncoroni, C.; Crugnola, A.; Yokota, T.; Bredesen, D.E.; Volterra, A. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 2008, 15, 1691–1700. [Google Scholar] [CrossRef] [Green Version]
- Anneser, J.; Chahli, C.; Borasio, G. Protective effect of metabotropic glutamate receptor inhibition on amyotrophic lateral sclerosis–cerebrospinal fluid toxicity in vitro. Neuroscience 2006, 141, 1879–1886. [Google Scholar] [CrossRef]
- Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci. 2019, 12, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrinmayeh, H.; Territo, P.R. Purinergic Receptors of the Central Nervous System: Biology, PET Ligands, and Their Applications. Mol. Imaging 2020, 19, 1536012120927609. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Segala, E.; Guo, D.; Cheng, R.K.Y.; Bortolato, A.; Deflorian, F.; Doré, A.S.; Errey, J.C.; Heitman, L.H.; Ijzerman, A.P.; Marshall, F.H.; et al. Controlling the Dissociation of Ligands from the Adenosine A2A Receptor through Modulation of Salt Bridge Strength. J. Med. Chem. 2016, 59, 6470–6479. [Google Scholar] [CrossRef]
- Chemical Computing Group ULC. Molecular Operating Environment (MOE); Chemical Computing Group ULC: Montreal, QC, Canada, 2021. [Google Scholar]
- Burnstock, G. Purinergic Signalling: Therapeutic Developments. Front. Pharmacol. 2017, 8, 661. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, J.; Gao, Z.-G.; Zhang, D.; Zhu, L.; Han, G.W.; Moss, S.M.; Paoletta, S.; Kiselev, E.; Lu, W.; et al. Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature 2014, 509, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Agle, K.A.; Vongsa, R.A.; Dwinell, M.B. Calcium Mobilization Triggered by the Chemokine CXCL12 Regulates Migration in Wounded Intestinal Epithelial Monolayers. J. Biol. Chem. 2010, 285, 16066–16075. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Wu, L.; Yuan, S.; Wu, M.; Xu, Y.; Sun, Q.; Li, S.; Zhao, S.; Hua, T.; Liu, Z.-J. Structural basis of CXC chemokine receptor 2 activation and signalling. Nature 2020, 585, 135–140. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589, PMCID:PMC8371605. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chien, E.Y.T.; Mol, C.D.; Fenalti, G.; Liu, W.; Katritch, V.; Abagyan, R.; Brooun, A.; Wells, P.; Bi, F.C.; et al. Structures of the CXCR4 Chemokine GPCR with Small-Molecule and Cyclic Peptide Antagonists. Science 2010, 330, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.D.; Karnik, S.S. Angiotensin Receptors: Structure, Function, Signaling and Clinical Applications. J. Cell Signal. 2016, 1, 111. [Google Scholar] [CrossRef] [PubMed]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Veter. Intern. Med. 2018, 33, 363–382. [Google Scholar] [CrossRef] [Green Version]
- Miura, S.-I.; Karnik, S.S.; Saku, K. Review: Angiotensin II type 1 receptor blockers: Class effects versus molecular effects. J. Renin-Angiotensin-Aldosterone Syst. 2010, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Akishita, M.; Ito, M.; Lehtonen, J.Y.; Daviet, L.; Dzau, V.J.; Horiuchi, M. Expression of the AT2 receptor developmentally programs extracellular signal-regulated kinase activity and influences fetal vascular growth. J. Clin. Investig. 1999, 103, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Unal, H.; Desnoyer, R.; Han, G.W.; Patel, N.; Katritch, V.; Karnik, S.S.; Cherezov, V.; Stevens, R.C. Structural Basis for Ligand Recognition and Functional Selectivity at Angiotensin Receptor. J. Biol. Chem. 2015, 290, 29127–29139. [Google Scholar] [CrossRef] [Green Version]
- Perryman, R. Inhibition of the angiotensin II type 2 receptor AT2R is a novel therapeutic strategy for glioblastoma. Unpublished Work. 2022. [Google Scholar]
- Beaulieu, J.-M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallone, D.; Picetti, R.; Borrelli, E. Structure and function of dopamine receptors. Neurosci. Biobehav. Rev. 2000, 24, 125–132. [Google Scholar] [CrossRef]
- Zhuang, Y.; Xu, P.; Mao, C.; Wang, L.; Krumm, B.; Zhou, X.E.; Huang, S.; Liu, H.; Cheng, X.; Huang, X.-P.; et al. Structural insights into the human D1 and D2 dopamine receptor signaling complexes. Cell 2021, 184, 931–942.e18. [Google Scholar] [CrossRef]
- Xu, P.; Huang, S.; Mao, C.; Krumm, B.E.; Zhou, X.E.; Tan, Y.; Huang, X.-P.; Liu, Y.; Shen, D.-D.; Jiang, Y.; et al. Structures of the human dopamine D3 receptor-Gi complexes. Mol. Cell 2021, 81, 1147–1159.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wacker, D.; Levit, A.; Che, T.; Betz, R.M.; McCorvy, J.D.; Venkatakrishnan, A.J.; Huang, X.-P.; Dror, R.O.; Shoichet, B.K.; et al. D4 dopamine receptor high-resolution structures enable the discovery of selective agonists. Science 2017, 358, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.E.; Nichols, C.D. Serotonin Receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Frazer, A.; Hensler, J.G. Serotonin receptors. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Siegel, G.J., Agranoff, B.W., Albers, R.W., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Xu, P.; Huang, S.; Zhang, H.; Mao, C.; Zhou, X.E.; Cheng, X.; Simon, I.A.; Shen, D.-D.; Yen, H.-Y.; Robinson, C.V.; et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 2021, 592, 469–473. [Google Scholar] [CrossRef] [PubMed]
- McCorvy, J.D.; Wacker, D.; Wang, S.; Agegnehu, B.; Liu, J.; Lansu, K.; Tribo, A.R.; Olsen, R.H.J.; Che, T.; Jin, J.; et al. Structural determinants of 5-HT2B receptor activation and biased agonism. Nat. Struct. Mol. Biol. 2018, 25, 787–796. [Google Scholar] [CrossRef]
- Peng, Y.; McCorvy, J.D.; Harpsøe, K.; Lansu, K.; Yuan, S.; Popov, P.; Qu, L.; Pu, M.; Che, T.; Nikolajsen, L.F.; et al. 5-HT2C Receptor Structures Reveal the Structural Basis of GPCR Polypharmacology. Cell 2018, 172, 719–730.e14. [Google Scholar] [CrossRef] [Green Version]
- Marucci, G.; Ben, D.D.; Lambertucci, C.; Navia, A.M.; Spinaci, A.; Volpini, R.; Buccioni, M. GPR17 receptor modulators and their therapeutic implications: Review of recent patents. Expert Opin. Ther. Patents 2019, 29, 85–95. [Google Scholar] [CrossRef]
- Dziedzic, A.; Miller, E.; Saluk-Bijak, J.; Bijak, M. The GPR17 Receptor—A Promising Goal for Therapy and a Potential Marker of the Neurodegenerative Process in Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 1852. [Google Scholar] [CrossRef] [Green Version]
- Marucci, G.; Ben, D.D.; Lambertucci, C.; Santinelli, C.; Spinaci, A.; Thomas, A.; Volpini, R.; Buccioni, M. The G Protein-Coupled Receptor GPR17: Overview and Update. ChemMedChem 2016, 11, 2567–2574. [Google Scholar] [CrossRef]
- Insel, P.A. Adrenergic Receptors—Evolving Concepts and Clinical Implications. N. Engl. J. Med. 1996, 334, 580–585. [Google Scholar] [CrossRef]
- Subbarao, K.V.; Hertz, L. Effect of adrenergic agonists on glycogenolysis in primary cultures of astrocytes. Brain Res. 1990, 536, 220–226. [Google Scholar] [CrossRef]
- Patel, M.; Shaw, D. A review of standard pharmacological therapy for adult asthma—Steps 1 to 5. Chronic Respir. Dis. 2015, 12, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ling, S.; Zhou, Y.; Zhang, Y.; Lv, P.; Liu, S.; Fang, W.; Sun, W.; A Hu, L.; Zhang, L.; et al. Different conformational responses of the β2-adrenergic receptor-Gs complex upon binding of the partial agonist salbutamol or the full agonist isoprenaline. Natl. Sci. Rev. 2020, 8. [Google Scholar] [CrossRef]
- Parsons, M.E.; Ganellin, C.R. Histamine and its receptors. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S127–S135. [Google Scholar] [CrossRef] [Green Version]
- Tiligada, E.; Ennis, M. Histamine pharmacology: From Sir Henry Dale to the 21st century. J. Cereb. Blood Flow Metab. 2018, 177, 469–489. [Google Scholar] [CrossRef] [Green Version]
- Xia, R.; Wang, N.; Xu, Z.; Lu, Y.; Song, J.; Zhang, A.; Guo, C.; He, Y. Cryo-EM structure of the human histamine H1 receptor/Gq complex. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- DeepMind. The AlphaFold Database. Available online: https://alphafold.ebi.ac.uk/ (accessed on 22 March 2022).
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, N.E. Immune regulation by cannabinoid compounds through the inhibition of the cyclic AMP signaling cascade and altered gene expression. Biochem. Pharmacol. 1996, 52, 1133–1140. [Google Scholar] [CrossRef]
- Bilsland, L.G.; Dick, J.R.T.; Pryce, G.; Petrosino, S.; Di Marzo, V.; Baker, D.; Greensmith, L. Increasing cannabinoid levels by pharmacological and genetic manipulation delays disease progression in SOD1 mice. FASEB J. 2006, 20, 1003–1005. [Google Scholar] [CrossRef]
- Hua, T.; Li, X.; Wu, L.; Iliopoulos-Tsoutsouvas, C.; Wang, Y.; Wu, M.; Shen, L.; Brust, C.A.; Nikas, S.P.; Song, F.; et al. Activation and Signaling Mechanism Revealed by Cannabinoid Receptor-Gi Complex Structures. Cell 2020, 180, 655–665.e18. [Google Scholar] [CrossRef]
- Xing, C.; Zhuang, Y.; Xu, T.-H.; Feng, Z.; Zhou, X.E.; Chen, M.; Wang, L.; Meng, X.; Xue, Y.; Wang, J.; et al. Cryo-EM Structure of the Human Cannabinoid Receptor CB2-Gi Signaling Complex. Cell 2020, 180, 645–654.e13. [Google Scholar] [CrossRef]
- Reader, J.; Holt, D.; Fulton, A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev. 2011, 30, 449–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, T.; Narumiya, S.; Zeilhofer, H.U. Contribution of peripheral versus central EP1 prostaglandin receptors to inflammatory pain. Neurosci. Lett. 2011, 495, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Fedyk, E.R.; Phipps, R.P. Prostaglandin E2 receptors of the EP2 and EP4 subtypes regulate activation and differentiation of mouse B lymphocytes to IgE-secreting cells. Proc. Natl. Acad. Sci. USA 1996, 93, 10978–10983. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Kawamori, T.; Nakatsugi, S.; Ohta, T.; Ohuchida, S.; Yamamoto, H.; Maruyama, T.; Kondo, K.; Ushikubi, F.; Narumiya, S.; et al. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res. 1999, 59, 5093–5096. [Google Scholar] [PubMed]
- Sun, X.; Li, Q. Prostaglandin EP2 receptor: Novel therapeutic target for human cancers (Review). Int. J. Mol. Med. 2018, 42, 1203–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, K.; Kato, S.; Amagase, K. Prostaglandin EP Receptors Involved in Modulating Gastrointestinal Mucosal Integrity. J. Pharmacol. Sci. 2010, 114, 248–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Du, Y. Distinct Roles of Central and Peripheral Prostaglandin E2 and EP Subtypes in Blood Pressure Regulation. Am. J. Hypertens. 2012, 25, 1042–1049. [Google Scholar] [CrossRef] [Green Version]
- Friedman, E.A.; Ogletree, M.L.; Haddad, E.V.; Boutaud, O. Understanding the role of prostaglandin E2 in regulating human platelet activity in health and disease. Thromb. Res. 2015, 136, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Takasaki, I.; Nojima, H.; Shiraki, K.; Sugimoto, Y.; Ichikawa, A.; Ushikubi, F.; Narumiya, S.; Kuraishi, Y. Involvement of cyclooxygenase-2 and EP3 prostaglandin receptor in acute herpetic but not postherpetic pain in mice. Neuropharmacology 2005, 49, 283–292. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, W.; Ge, J.; Zhang, Z. Prostaglandin E2 receptor EP4 is involved in the cell growth and invasion of prostate cancer via the cAMP-PKA/PI3K-Akt signaling pathway. Mol. Med. Rep. 2018, 17, 4702–4712. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Mao, C.; Xiao, P.; Shen, Q.; Zhong, Y.-N.; Yang, F.; Shen, D.-D.; Tao, X.; Zhang, H.; Yan, X.; et al. Ligand recognition, unconventional activation, and G protein coupling of the prostaglandin E2 receptor EP2 subtype. Sci. Adv. 2021, 7, eabf1268. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Suno, R.; Hotta, Y.; Yamashita, K.; Hirata, K.; Yamamoto, M.; Narumiya, S.; Iwata, S.; Kobayashi, T. Crystal structure of the endogenous agonist-bound prostanoid receptor EP3. Nat. Chem. Biol. 2018, 15, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Nojima, S.; Fujita, Y.; Kimura, K.T.; Nomura, N.; Suno, R.; Morimoto, K.; Yamamoto, M.; Noda, T.; Iwata, S.; Shigematsu, H.; et al. Cryo-EM Structure of the Prostaglandin E Receptor EP4 Coupled to G Protein. Structure 2020, 29, 252–260.e6. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, Y.; Tenno, T.; Goda, N.; Shirakawa, M.; Ikegami, T.; Hiroaki, H. Structural difference of vasoactive intestinal peptide in two distinct membrane-mimicking environments. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2011, 1814, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Harmar, A.J.; Fahrenkrug, J.; Gozes, I.; Laburthe, M.; May, V.; Pisegna, J.R.; Vaudry, D.; Vaudry, H.; A Waschek, J.; I Said, S. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR Review 1. J. Cereb. Blood Flow Metab. 2012, 166, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Couvineau, A.; Laburthe, M. VPAC receptors: Structure, molecular pharmacology and interaction with accessory proteins. J. Cereb. Blood Flow Metab. 2012, 166, 42–50. [Google Scholar] [CrossRef]
- Duan, J.; Shen, D.-D.; Zhou, X.E.; Bi, P.; Liu, Q.-F.; Tan, Y.-X.; Zhuang, Y.-W.; Zhang, H.-B.; Xu, P.-Y.; Huang, S.-J.; et al. Cryo-EM structure of an activated VIP1 receptor-G protein complex revealed by a NanoBiT tethering strategy. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Qu, L.; Wu, L.; Tang, X.; Luo, F.; Xu, W.; Xu, Y.; Liu, Z.-J.; Hua, T. Structural insights into the activation initiation of full-length mGlu1. Protein Cell 2020, 12, 662–667. [Google Scholar] [CrossRef]
- Seven, A.B.; Barros-Álvarez, X.; de Lapeyrière, M.; Papasergi-Scott, M.M.; Robertson, M.J.; Zhang, C.; Nwokonko, R.M.; Gao, Y.; Meyerowitz, J.G.; Rocher, J.-P.; et al. G-protein activation by a metabotropic glutamate receptor. Nature 2021, 595, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Han, S.; Cai, X.; Tan, Q.; Zhou, K.; Wang, D.; Wang, X.; Du, J.; Yi, C.; Chu, X.; et al. Structures of Gi-bound metabotropic glutamate receptors mGlu2 and mGlu4. Nature 2021, 594, 583–588. [Google Scholar] [CrossRef] [PubMed]
























| Molecule | Target/Mechanism | Developer | Clinical Phase |
|---|---|---|---|
| Ibudilast | Macrophage migration inhibitory factor inhibitor | MediciNova | Phase II/III |
| Prosetin | Mitogen-activated protein kinase inhibitor | ProJenX | Phase I |
| Sotuletinib | Macrophage colony-stimulating factor receptor antagonist | Novartis | Phase II |
| EPI 589 | NAD(P)H dehydrogenase modulator | PTC Therapeutics | Phase II |
| DNL 343 | Eukaryoticinitiationfactor2b stimulant | Denali Therapeutics Inc | Phase I |
| Celecoxib/ciprofloxacin | Cyclo-oxygenase 2 inhibitors/DNA gyrase inhibitors | NeuroSense Therapeutics | Phase I |
| Fingolimod | Apoptosis stimulant and immunosuppressant | ALS Therapy Development Institute | Phase II |
| Trehalose | Autophagy stimulant and protein aggregation inhibitor | Massachusetts General Hospital | Phase II/III |
| Sodium cromoglicate | Glial cell modulator and mast cell stabilizer | AZTherapies | Phase II |
| Dexpramipexole | Antioxidant and apoptosis inhibitor | Knopp Biosciences | Phase II |
| Masitinib | Tyrosine kinase inhibitor | AB Science | Phase III |
| NP 001 | Macrophage modulator | Neuvivo | Phase II |
| Fasudil | Rho-associated kinase inhibitor and vasodilatator | Woolsey Pharmaceuticals | Phase II |
| Levosimendan | Calcium-sensitising phosphodiesterase inhibitor and potassium channel agonist | Orion | Phase III |
| Apilimoddimesylate | Interleukin 12 inhibitor and interleukin 23 inhibitor | AI Therapeutics | Phase II |
| Verdiperstat | Peroxidase inhibitor | Biohaven Pharmaceuticals | Phase II/III |
| Pridopidine | Sigma-1 receptor agonist | Massachusetts General Hospital, Prilenia Therapeutics | Phase II/III |
| Triheptanoin | Triglyceride replacement agent | Ultragenyx Pharmaceutical | Phase I/II |
| Reldesemtiv | Troponin stimulant | Cytokinetics | Phase III |
| BIIB 100 | Exportin-1 protein inhibitor | Biogen | Phase I |
| AGX 201 | Histamine receptor modulator | AgoneX Biopharmaceuticals | Phase I |
| Ranolazine extended release | Sodium channel antagonist | Gilead Sciences | Phase II |
| GDC 0134 | Mitogen-activated protein kinase 12 inhibitor | Genentech | Phase I |
| NPT520 34 | Phosphatidylinositol 3 kinase modulator | Neuropore Therapies | Phase I |
| Receptor/Receptor Family | Cellular Expression | Potential for ALS Treatment | References |
|---|---|---|---|
| Adenosine receptors | Circulatory, immune, respiratory, and nervous systems | Ambiguous | [30,31,32,33,34,35,36,37] |
| Purinergic receptors P2Y | Almost all human tissues | Antagonism | [38,39,40,41,42,43] |
| Chemokine receptors | Predominantly on leukocytes surface | CXCR3, CXCR4, and CCR2 Antagonism | [44,45,46,47] |
| Angiotensin II receptors | Adrenal cortex, kidneys, vascular and cardiac muscles, nervous system | AT1 Antagonism | [48,49,50] |
| Dopamine receptors | Arteries, heart, kidneys, CNS | D2R Agonism | [51,52,53,54,55,56,57,58] |
| Serotonin receptors | Almost all human tissues | Ambiguous | [59,60,61,62,63] |
| GPR17 receptor | CNS, kidneys, heart | Antagonism | [64,65,66,67,68,69,70,71] |
| Adrenergic receptor β2 | GI tract, respiratory system, blood vessels, pancreas, nervous system | Agonism | [72,73] |
| Histamine receptors | GI tract, circulatory, immune, and nervous systems. | Ambiguous | [74,75,76,77] |
| Cannabinoid receptors | CNS and immune system | CB2 agonism | [78,79,80,81] |
| Prostaglandin E2 receptor | GI tract, kidneys, reproductive, skeletal, immune, and nervous systems. | Ambiguous | [82,83,84] |
| Vasoactive intestinal peptide receptors | Almost all human tissues | Agonism | [85,86,87,88,89] |
| Metabotropic glutamate receptors | Nervous system | mGluR I antagonism/mGluR II and mGluR III agonism | [90,91,92,93,94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassani, D.; Pavan, M.; Federico, S.; Spalluto, G.; Sturlese, M.; Moro, S. The Multifaceted Role of GPCRs in Amyotrophic Lateral Sclerosis: A New Therapeutic Perspective? Int. J. Mol. Sci. 2022, 23, 4504. https://doi.org/10.3390/ijms23094504
Bassani D, Pavan M, Federico S, Spalluto G, Sturlese M, Moro S. The Multifaceted Role of GPCRs in Amyotrophic Lateral Sclerosis: A New Therapeutic Perspective? International Journal of Molecular Sciences. 2022; 23(9):4504. https://doi.org/10.3390/ijms23094504
Chicago/Turabian StyleBassani, Davide, Matteo Pavan, Stephanie Federico, Giampiero Spalluto, Mattia Sturlese, and Stefano Moro. 2022. "The Multifaceted Role of GPCRs in Amyotrophic Lateral Sclerosis: A New Therapeutic Perspective?" International Journal of Molecular Sciences 23, no. 9: 4504. https://doi.org/10.3390/ijms23094504