Unraveling the Role of Sex Hormones on Keratinocyte Functions in Human Inflammatory Skin Diseases
Abstract
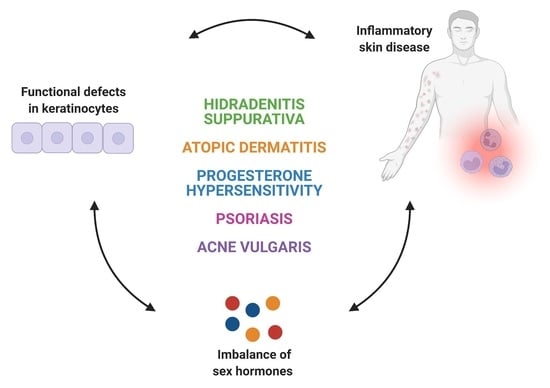
:1. Introduction
1.1. The Skin
1.2. Cutaneous Immune Responses
Role of Keratinocytes in Skin Immunity
1.3. The Skin as a Target of Hormones and as an Independent Peripheral Endocrine Organ
1.3.1. The Skin as a Target Site for Hormones
1.3.2. The Skin as an Independent Peripheral Endocrine Organ
1.3.3. Role of Sex Hormones on Human Skin
2. Inflammatory Skin Diseases with Alterations in Keratinocyte Function and Sex Hormones Imbalance
2.1. Hidradenitis Suppurativa
2.2. Acne Vulgaris
2.3. Atopic Dermatitis
2.4. Progesterone Hypersensitivity
2.5. Psoriasis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zouboulis, C.P.D. Human Skin: An Independent Peripheral Endocrine Organ. Horm. Res. Paediatr. 2000, 54, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and Function of the Skin. In Nanoscience in Dermatology; Elsevier BV-Academic Press: London, UK; Oxford, UK; San Diego, CA, USA; Cambridge, MA, USA, 2016; pp. 1–14. ISBN 978-0-12-802926-8. [Google Scholar]
- Gratton, R.; Tricarico, P.M.; Moltrasio, C.; De Oliveira, A.S.L.E.; Brandão, L.; Marzano, A.V.; Zupin, L.; Crovella, S. Pleiotropic Role of Notch Signaling in Human Skin Diseases. Int. J. Mol. Sci. 2020, 21, 4214. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C. The skin as an endocrine organ. Dermato-Endocrinol. 2009, 1, 250–252. [Google Scholar] [CrossRef]
- Albanesi, C.; Scarponi, C.; Giustizieri, M.L.; Girolomoni, G. Keratinocytes in Inflammatory Skin Diseases. Curr. Drug Targets Inflamm. Allergy 2005, 4, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M. Mammalian skin cell biology: At the interface between laboratory and clinic. Science 2014, 346, 937–940. [Google Scholar] [CrossRef]
- Sabat, R.; Jemec, G.B.E.; Matusiak, Ł.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Prim. 2020, 6, 18. [Google Scholar] [CrossRef]
- Briganti, S.; Flori, E.; Mastrofrancesco, A.; Ottaviani, M. Acne as an altered dermato-endocrine response problem. Exp. Dermatol. 2020, 29, 833–839. [Google Scholar] [CrossRef]
- Irvine, A.; McLean, W.H.I.; Leung, D.Y. Filaggrin Mutations Associated with Skin and Allergic Diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef] [Green Version]
- Buerger, C.; Shirsath, N.; Lang, V.; Berard, A.; Diehl, S.; Kaufmann, R.; Boehncke, W.-H.; Wolf, P. Inflammation dependent mTORC1 signaling interferes with the switch from keratinocyte proliferation to differentiation. PLoS ONE 2017, 12, e0180853. [Google Scholar] [CrossRef] [Green Version]
- Kupper, T.S.; Fuhlbrigge, R.C. Immune surveillance in the skin: Mechanisms and clinical consequences. Nat. Rev. Immunol. 2004, 4, 211–222. [Google Scholar] [CrossRef]
- Takashima, A. Cytokine-mediated communication by keratinocytes and Langerhans cells with dendritic epidermal T cells. Semin. Immunol. 1996, 8, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kupper, T.S.; Groves, R.W. The Interleukin-1 Axis and Cutaneous Inflammation. J. Investig. Dermatol. 1995, 105, 62S–66S. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Egawa, G.; Kabashima, K. Antigen presentation and adaptive immune responses in skin. Int. Immunol. 2019, 31, 423–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestrallet, G.; Rouas-Freiss, N.; LeMaoult, J.; Fortunel, N.O.; Martin, M.T. Skin Immunity and Tolerance: Focus on Epidermal Keratinocytes Expressing HLA-G. Front. Immunol. 2021, 12, 772516. [Google Scholar] [CrossRef]
- Hirai, T.; Whitley, S.K.; Kaplan, D.H. Migration and Function of Memory CD8+ T Cells in Skin. J. Investig. Dermatol. 2019, 140, 748–755. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Nickoloff, B.J. Keratinocyte Intercellular Adhesion Molecule-1 (ICAM-1) Expression Precedes Dermal T Lym-phocytic Infiltration in Allergic Contact Dermatitis (Rhus Dermatitis). Am. J. Pathol. 1989, 135, 1045–1053. [Google Scholar]
- Black, A.P.B.; Ardern-Jones, M.R.; Kasprowicz, V.; Bowness, P.; Jones, L.; Bailey, A.S.; Ogg, G.S. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur. J. Immunol. 2007, 37, 1485–1493. [Google Scholar] [CrossRef]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 2020, 5, 142067. [Google Scholar] [CrossRef]
- Than, U.; Leavesley, D.; Parker, T. Characteristics and roles of extracellular vesicles released by epidermal keratinocytes. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2264–2272. [Google Scholar] [CrossRef]
- Klicznik, M.; Szenes-Nagy, A.; Campbell, D.; Gratz, I. Taking the lead—How keratinocytes orchestrate skin T cell immunity. Immunol. Lett. 2018, 200, 43–51. [Google Scholar] [CrossRef]
- Foster, C.A.; Yokozeki, H.; Rappersberger, K.; Koning, F.; Volc-Platzer, B.; Rieger, A.; Coligan, J.E.; Wolff, K.; Stingl, G. Human epidermal T cells predominantly belong to the lineage expressing alpha/beta T cell receptor. J. Exp. Med. 1990, 171, 997–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.C.; Tan, X.-Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reyero, N. The clandestine organs of the endocrine system. Gen. Comp. Endocrinol. 2018, 257, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, C.; Hendry, C.; Farley, A.; McLafferty, E. Endocrine system: Part 1. Nurs. Stand. 2014, 28, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Deplewski, D.; Rosenfield, R.L. Role of Hormones in Pilosebaceous Unit Development. Endocr. Rev. 2000, 21, 363–392. [Google Scholar] [CrossRef]
- Zouboulis, C.C. The human skin as a hormone target and an endocrine gland. Hormones 2004, 3, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Haczynski, J.; Tarkowski, R.; Jarzabek, K.; Slomczynska, M.; Wolczynski, S.; Magoffin, D.A.; Jakowicki, J.A.; Jakimiuk, A.J. Human cultured skin fibroblasts express estrogen receptor alpha and beta. Int. J. Mol. Med. 2002, 10, 149–153. [Google Scholar]
- Chen, W.; Thiboutot, D.; Zouboulis, C.P.D. Cutaneous Androgen Metabolism: Basic Research and Clinical Perspectives. J. Investig. Dermatol. 2002, 119, 992–1007. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef]
- Navarro-Triviño, F.; Arias-Santiago, S.; Gilaberte-Calzada, Y. Vitamina D y la piel. Una revisión para dermatólogos. Actas Dermo-Sifiliográficas 2019, 110, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Lehmann, B.; Carlberg, C.; Varani, J.; Zouboulis, C.P.D. Vitamins as Hormones. Horm. Metab. Res. 2007, 39, 71–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouboulis, C.C.; Degitz, K. Androgen action on human skin—From basic research to clinical significance. Exp. Dermatol. 2004, 13 (Suppl. S4), 5–10. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. The role of estrogen in cutaneous ageing and repair. Maturitas 2017, 103, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Choi, H.; Kim, B.; Kim, M.; Park, K.-N.; Bae, I.-H.; Sung, Y.K.; Lee, T.R.; Shin, D.W.; Bae, Y.S. Testosterone Stimulates Duox1 Activity through GPRC6A in Skin Keratinocytes. J. Biol. Chem. 2014, 289, 28835–28845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, S.; Werz, O. Impact of Androgens on Inflammation-Related Lipid Mediator Biosynthesis in Innate Immune Cells. Front. Immunol. 2020, 11, 1356. [Google Scholar] [CrossRef]
- Traish, A.; Bolanos, J.; Nair, S.; Saad, F.; Morgentaler, A. Do Androgens Modulate the Pathophysiological Pathways of Inflammation? Appraising the Contemporary Evidence. J. Clin. Med. 2018, 7, 549. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.-J.; Chang, P.; Lai, K.-P.; Chen, L.; Chang, C. The role of androgen and androgen receptor in skin-related disorders. Arch. Dermatol. Res. 2012, 304, 499–510. [Google Scholar] [CrossRef]
- Verdier-Sevrain, S.; Bonte, F.; Gilchrest, B. Biology of estrogens in skin: Implications for skin aging. Exp. Dermatol. 2006, 15, 83–94. [Google Scholar] [CrossRef]
- Bereshchenko, O.; Bruscoli, S.; Riccardi, C. Glucocorticoids, Sex Hormones, and Immunity. Front. Immunol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, Z.; Chen, Y.; Chen, Y.; Huang, Z.; You, B.; Peng, Y.; Chen, J. Estrogen Accelerates Cutaneous Wound Healing by Promoting Proliferation of Epidermal Keratinocytes via Erk/Akt Signaling Pathway. Cell. Physiol. Biochem. 2016, 38, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Cario, M. How hormones may modulate human skin pigmentation in melasma: An in vitro perspective. Exp. Dermatol. 2019, 28, 709–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asavasupreechar, T.; Saito, R.; Miki, Y.; Edwards, D.P.; Boonyaratanakornkit, V.; Sasano, H. Systemic distribution of progesterone receptor subtypes in human tissues. J. Steroid Biochem. Mol. Biol. 2020, 199, 105599. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Mace, B.; Dawson, H.N.; Warner, D.S.; Laskowitz, D.T.; James, M.L. Anti-Inflammatory Effects of Progesterone in Lipopolysaccharide-Stimulated BV-2 Microglia. PLoS ONE 2014, 9, e103969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, K.; Chen, L.; Georgiou, E.X.; Sooranna, S.R.; Khanjani, S.; Brosens, J.J.; Bennett, P.R.; Johnson, M.R. Progesterone Acts via the Nuclear Glucocorticoid Receptor to Suppress IL-1β-Induced COX-2 Expression in Human Term Myometrial Cells. PLoS ONE 2012, 7, e50167. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.-T.; Kou, X.-X.; Li, C.; Bi, R.-Y.; Meng, Z.; Wang, X.-D.; Zhou, Y.-H.; Gan, Y.-H. Progesterone attenuates temporomandibular joint inflammation through inhibition of NF-κB pathway in ovariectomized rats. Sci. Rep. 2017, 7, 15334. [Google Scholar] [CrossRef] [Green Version]
- Kanda, N.; Watanabe, S. Regulatory roles of sex hormones in cutaneous biology and immunology. J. Dermatol. Sci. 2005, 38, 1–7. [Google Scholar] [CrossRef]
- Im, S.; Lee, E.S.; Kim, W.; Song, J.; Kim, J.; Lee, M.; Kang, W.H. Expression of progesterone receptor in human keratinocytes. J. Korean Med. Sci. 2000, 15, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Vossen, A.R.J.V.; Van Der Zee, H.H.; Prens, E.P. Hidradenitis Suppurativa: A Systematic Review Integrating Inflammatory Pathways into a Cohesive Pathogenic Model. Front. Immunol. 2018, 9, 2965. [Google Scholar] [CrossRef] [Green Version]
- Riis, P.T.; Ring, H.C.; Themstrup, L.; Jemec, G.B. The Role of Androgens and Estrogens in Hidradenitis Suppurativa—A Sys-tematic Review. Acta Dermatovenerol. Croat. 2016, 24, 239–249. [Google Scholar]
- Harrison, B.J.; Read, G.F.; Hughes, L.E. Endocrine basis for the clinical presentation of hidradenitis suppurativa. Br. J. Surg. 2005, 75, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Ring, H.C.; Riis, P.T.; Zarchi, K.; Miller, I.M.; Saunte, D.M.; Jemec, G.B. Prodromal symptoms in hidradenitis suppurativa. Clin. Exp. Dermatol. 2017, 42, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Collier, E.; Shi, V.Y.; Parvataneni, R.K.; Lowes, M.A.; Hsiao, J.L. Special considerations for women with hidradenitis suppurativa. Int. J. Women’s Dermatol. 2020, 6, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Neuren, E.; Strunk, A. Hidradenitis Suppurativa Is Associated with Polycystic Ovary Syndrome: A Population-Based Analysis in the United States. J. Investig. Dermatol. 2018, 138, 1288–1292. [Google Scholar] [CrossRef] [Green Version]
- Revuz, J.E.; Canoui-Poitrine, F.; Wolkenstein, P.; Viallette, C.; Gabison, G.; Pouget, F.; Poli, F.; Faye, O.; Roujeau, J.C.; Bonnelye, G.; et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J. Am. Acad. Dermatol. 2008, 59, 596–601. [Google Scholar] [CrossRef]
- Mirmirani, P.; Carpenter, D.M. Skin Disorders Associated with Obesity in Children and Adolescents: A Population-Based Study. Pediatr. Dermatol. 2014, 31, 183–190. [Google Scholar] [CrossRef]
- Buonomo, M.; Mansh, M.; Thorpe, D.; Goldfarb, N. Development or exacerbation of hidradenitis suppurativa in two transgender men after initiation of testosterone therapy. Br. J. Dermatol. 2021, 184, 1192–1194. [Google Scholar] [CrossRef]
- Lai, J.-J.; Lai, K.-P.; Chuang, K.-H.; Chang, P.; Yu, I.-C.; Lin, W.-J.; Chang, C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-α expression. J. Clin. Investig. 2009, 119, 3739–3751. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Barrett, J.; Liu, P.; Parameswaran, A.; Chiu, E.; Lu, C. Novel evidence of androgen receptor immunoreactivity in skin tunnels of hidradenitis suppurativa: Assessment of sex and individual variability. Br. J. Dermatol. 2021, 185, 855–858. [Google Scholar] [CrossRef]
- Gauntner, T.D. Hormonal, stem cell and Notch signalling as possible mechanisms of disease in hidradenitis suppurativa: A systems-level transcriptomic analysis. Br. J. Dermatol. 2019, 180, 203–204. [Google Scholar] [CrossRef] [Green Version]
- Zouboulis, C.C.; Da Costa, A.N.; Fimmel, S.; Zouboulis, K.C. Apocrine glands are bystanders in hidradenitis suppurativa and their involvement is gender specific. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1555–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buimer, M.G.; Wobbes, T.; Klinkenbijl, J.H.G.; Reijnen, M.M.P.J.; Blokx, W.A.M. Immunohistochemical Analysis of Steroid Hormone Receptors in Hidradenitis Suppurativa. Am. J. Dermatopathol. 2015, 37, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Kuttenn, F.; Mowszowicz, I.; Schaison, G.; Mauvais-Jarvis, P. Androgen Production and Skin Metabolism in Hirsutism. J. Endocrinol. 1977, 75, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.N.; Searles, G.E. Hidradenitis Suppurativa in 64 Female Patients: Retrospective Study Comparing Oral Antibiotics and Antiandrogen Therapy. J. Cutan. Med. Surg. 2007, 11, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.; Layton, A.; Cunliffe, W. Endocrine factors in pre- and postmenopausal women with hidradenitis suppurativa. Br. J. Dermatol. 1996, 134, 1057–1059. [Google Scholar] [CrossRef]
- Rao, A.; Douglas, S.; Hall, J. Endocrine Disrupting Chemicals, Hormone Receptors, and Acne Vulgaris: A Connecting Hypothesis. Cells 2021, 10, 1439. [Google Scholar] [CrossRef]
- Tuchayi, S.M.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.P.D. Acne vulgaris. Nat. Rev. Dis. Prim. 2015, 1, 15029. [Google Scholar] [CrossRef]
- Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 8–12. [Google Scholar] [CrossRef]
- Bharti, S.; Vadlamudi, H.C. A strategic review on the involvement of receptors, transcription factors and hormones in acne pathogenesis. J. Recept. Signal Transduct. 2021, 41, 105–116. [Google Scholar] [CrossRef]
- Cong, T.-X.; Hao, D.; Wen, X.; Li, X.-H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef]
- Kumtornrut, C.; Yamauchi, T.; Koike, S.; Aiba, S.; Yamasaki, K. Androgens modulate keratinocyte differentiation indirectly through enhancing growth factor production from dermal fibroblasts. J. Dermatol. Sci. 2019, 93, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Med. Port. 2019, 32, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Watson, W.; Carr, S. Atopic dermatitis. Allergy Asthma Clin. Immunol. 2018, 14, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, T.; Honda, T.; Kabashima, K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int. Immunol. 2018, 30, 419–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokura, Y. Extrinsic and intrinsic types of atopic dermatitis. J. Dermatol. Sci. 2010, 58, 1–7. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. The Roles of Sex Hormones in the Course of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 4660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebata, T.; Itamura, R.; Aizawa, H.; Niimura, M. Serum Sex Hormone Levels in Adult Patients with Atopic Dermatitis. J. Dermatol. 1996, 23, 603–605. [Google Scholar] [CrossRef]
- Farage, M.A.; Neill, S.; MacLean, A.B. Physiological Changes Associated with the Menstrual Cycle. Obstet. Gynecol. Surv. 2009, 64, 58–72. [Google Scholar] [CrossRef]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. In Management of Atopic Dermatitis; Fortson, E.A., Feldman, S.R., Strowd, L.C., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 1027, pp. 21–37. ISBN 978-3-319-64803-3. [Google Scholar]
- Kemmett, D.; Tidman, M.J. The influence of the menstrual cycle and pregnancy on atopic dermatitis. Br. J. Dermatol. 1991, 125, 59–61. [Google Scholar] [CrossRef]
- Hannen, R.F.; Michael, A.E.; Jaulim, A.; Bhogal, R.; Burrin, J.M.; Philpott, M.P. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem. Biophys. Res. Commun. 2011, 404, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, K.M.; Bernstein, J.A. Progestogen Hypersensitivity: Heterogeneous Manifestations with a Common Trigger. J. Allergy Clin. Immunol. Pr. 2017, 5, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.L.; Carr, T.F. Iatrogenic autoimmune progesterone dermatitis treated with a novel intramuscular progesterone desensitization protocol. J. Allergy Clin. Immunol. Pract. 2013, 1, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Matsuda, M.; Yanbe, H.; Sugiyama, S. Two cases of autoimmune progesterone dermatitis. Immunohistochemical and serological studies. Acta Derm. Venereol. 1989, 69, 308–310. [Google Scholar] [PubMed]
- Meltzer, L. Hypersensitivity to Gonadal Hormones. South. Med. J. 1963, 56, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, A.; Vermorken, A.; Degreef, H.; Dooms-Goossens, A.; Vermorkkn, A. Corticosteroid or steroid allergy? Contact Dermat. 1992, 26, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Itsekson, A.M.; Lev-Sagie, A. Autoimmune Progesterone Dermatitis. Curr. Dermatol. Rep. 2013, 2, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Huang, T. Impact of pregnancy and oestrogen on psoriasis and potential therapeutic use of selective oestrogen receptor modulators for psoriasis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1085–1091. [Google Scholar] [CrossRef]
- Kanda, N.; Watanabe, S. 17β-Estradiol Stimulates the Growth of Human Keratinocytes by Inducing Cyclin D2 Expression. J. Investig. Dermatol. 2004, 123, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Merlo, S.; Frasca, G.; Canonico, P.L.; Sortino, M.A. Differential involvement of estrogen receptorα and estrogen receptorβ in the healing promoting effect of estrogen in human keratinocytes. J. Endocrinol. 2008, 200, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gratton, R.; Del Vecchio, C.; Zupin, L.; Crovella, S. Unraveling the Role of Sex Hormones on Keratinocyte Functions in Human Inflammatory Skin Diseases. Int. J. Mol. Sci. 2022, 23, 3132. https://doi.org/10.3390/ijms23063132
Gratton R, Del Vecchio C, Zupin L, Crovella S. Unraveling the Role of Sex Hormones on Keratinocyte Functions in Human Inflammatory Skin Diseases. International Journal of Molecular Sciences. 2022; 23(6):3132. https://doi.org/10.3390/ijms23063132
Chicago/Turabian StyleGratton, Rossella, Cecilia Del Vecchio, Luisa Zupin, and Sergio Crovella. 2022. "Unraveling the Role of Sex Hormones on Keratinocyte Functions in Human Inflammatory Skin Diseases" International Journal of Molecular Sciences 23, no. 6: 3132. https://doi.org/10.3390/ijms23063132