Synergetic Enhancement of Tumor Double-Targeted MRI Nano-Probe
Abstract
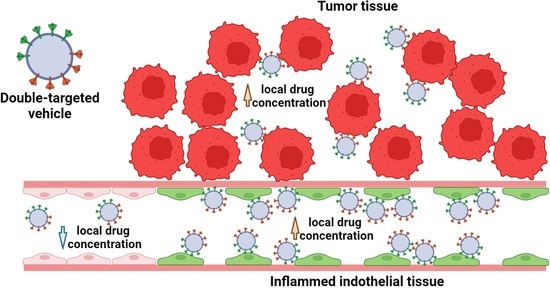
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PAMAM G4 Derivatives Synthesis
2.3. PAMAM G-4 PEG-Mal/OCH3 Coupling with IELLQARCRR and KQEFLINGCRR
2.4. Characterization
2.5. In Vitro Internalization Assay
2.6. Cytotoxicity Assay
2.7. MR Imaging
2.8. In Vivo and Ex Vivo Intratumoral Accumulation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Peptides-Conjugated PAMAM-PEG
3.2. Effects of Targeting Ligands on the Cellular Uptake
3.3. In Vivo Biodistribution and Intratumoral Accumulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Limeres, M.J.; Moretton, M.A.; Bernabeu, E.; Chiappetta, D.A.; Cuestas, M.L. Thinking small, doing big: Current success and future trends in drug delivery systems for improving cancer therapy with special focus on liver cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, D.P.; Otto, A.; de Villiers, M.M. Differences in physicochemical properties to consider in the design, evaluation and choice between microparticles and nanoparticles for drug delivery. Expert Opin. Drug Deliv. 2015, 12, 763–777. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, X.; Zhang, X.; Ma, L.; Wang, B.; Wei, H. Optimization of bioreducible micelles self-assembled from amphiphilic hyperbranched block copolymers for drug delivery. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1383–1394. [Google Scholar] [CrossRef] [Green Version]
- Pooja, D.; Srinivasa, R.T.; Kulhari, H.; Kadari, A.; Adams, D.J.; Bansal, V.; Sistla, R. N-acetyl-d-glucosamine-conjugated PAMAM dendrimers as dual receptor-targeting nanocarriers for anticancer drug delivery. Eur. J. Pharm. Biopharm. 2020, 154, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, C.; Zhang, Y.; Zheng, H.; Cheng, Y.; Zhu, J.; Chen, X.; Zhu, Z.; Piao, J.G.; Li, F. Targeted Manganese doped silica nano GSH-cleaner for treatment of Liver Cancer by destroying the intracellular redox homeostasis. Theranostics 2020, 10, 9865–9887. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Cell Selective Apoptosis Induced by Polymorphic Alteration of Self-Assembled Silica Nanowebs. ACS Appl. Mater. Interfaces 2017, 9, 6292–6305. [Google Scholar] [CrossRef]
- Wan, X.; Song, L.; Pan, W.; Zhong, H.; Li, N.; Tang, B. Tumor-Targeted Cascade Nanoreactor Based on Metal-Organic Frameworks for Synergistic Ferroptosis-Starvation Anticancer Therapy. ACS Nano 2020, 14, 11017–11028. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, S.; Zhang, N.; Kuang, Y.; Li, W.; Gai, S.; He, F.; Gulzar, A.; Yang, P. X-ray-triggered NO-released Bi-SNO nanoparticles: All-in-one nano-radiosensitizer with photothermal/gas therapy for enhanced radiotherapy. Nanoscale 2020, 12, 19293–19307. [Google Scholar] [CrossRef]
- Faustova, M.; Nikolskaya, E.; Sokol, M.; Zabolotsky, A.; Mollaev, M.; Zhunina, O.; Fomicheva, M.; Lobanov, A.; Severin, E.; Yabbarov, N. High-effective reactive oxygen species inducer based on Mn-tetraphenylporphyrin loaded PLGA nanoparticles in binary catalyst therapy. Free Radic. Biol. Med. 2019, 143, 522–533. [Google Scholar] [CrossRef]
- Sokol, M.B.; Yabbarov, N.G.; Mollaeva, M.R.; Chirkina, M.V.; Balabanyan, V.Y.; Nikolskaya, E.D. Development of the Composition and Technology for Obtaining Paclitaxel Nanoscale Formulation Consisting of a Conjugate of Polymer Particles with a Protein Vector Molecule. Drug Dev. Regist. 2021, 10, 81–88. [Google Scholar] [CrossRef]
- Mollaeva, M.R.; Yabbarov, N.; Sokol, M.; Chirkina, M.; Mollaev, M.D.; Zabolotskii, A.; Seregina, I.; Bolshov, M.; Kaplun, A.; Nikolskaya, E. Optimization, Characterization and Pharmacokinetic Study of Meso-Tetraphenylporphyrin Metal Complex-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12261. [Google Scholar] [CrossRef] [PubMed]
- Mollaeva, M.R.; Nikolskaya, E.; Beganovskaya, V.; Sokol, M.; Chirkina, M.; Obydennyi, S.; Belykh, D.; Startseva, O.; Mollaev, M.D.; Yabbarov, N. Oxidative Damage Induced by Phototoxic Pheophorbide a 17-Diethylene Glycol Ester Encapsulated in PLGA Nanoparticles. Antioxidants 2021, 10, 1985. [Google Scholar] [CrossRef]
- Sokol, M.B.; Nikolskaya, E.D.; Yabbarov, N.G.; Zenin, V.A.; Faustova, M.R.; Belov, A.V.; Zhunina, O.A.; Mollaev, M.D.; Zabolotsky, A.I.; Tereshchenko, O.G.; et al. Development of novel PLGA nanoparticles with co-encapsulation of docetaxel and abiraterone acetate for a highly efficient delivery into tumor cells. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, G.T.; Bracaglia, L.G.; Saltzman, W.M.; Pober, J.S. Focus on Fundamentals: Achieving Effective Nanoparticle Targeting. Trends Mol. Med. 2018, 24, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Mollaev, M.; Gorokhovets, N.; Nikolskaya, E.; Faustova, M.; Zabolotsky, A.; Zhunina, O.; Sokol, M.; Zamulaeva, I.; Severin, E.; Yabbarov, N. Type of pH sensitive linker reveals different time-dependent intracellular localization, in vitro and in vivo efficiency in alpha-fetoprotein receptor targeted doxorubicin conjugate. Int. J. Pharm. 2019, 559, 138–146. [Google Scholar] [CrossRef]
- Gonda, A.; Zhao, N.; Shah, J.V.; Calvelli, H.R.; Kantamneni, H.; Francis, N.L.; Ganapathy, V. Engineering Tumor-Targeting Nanoparticles as Vehicles for Precision Nanomedicine. Med. One 2019, 4, e190021. [Google Scholar]
- Thomas, O.S.; Weber, W. Overcoming Physiological Barriers to Nanoparticle Delivery-Are We There Yet? Front. Bioeng. Biotechnol. 2019, 7, 415. [Google Scholar] [CrossRef]
- Gao, J.Q.; Lv, Q.; Li, L.M.; Tang, X.J.; Li, F.Z.; Hu, Y.L.; Han, M. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials 2013, 34, 5628–5639. [Google Scholar] [CrossRef]
- Haense, N.; Atmaca, A.; Pauligk, C.; Steinmetz, K.; Marmé, F.; Haag, G.M.; Rieger, M.; Ottmann, O.G.; Ruf, P.; Lindhofer, H.; et al. A phase I trial of the trifunctional anti Her2 × anti CD3 antibody ertumaxomab in patients with advanced solid tumors. BMC Cancer 2016, 16, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.E.; Yumul, R.C.; Lin, J.; Cheng, Y.; Lieber, A.; Pun, S.H. Junction opener protein increases nanoparticle accumulation in solid tumors. J. Control. Release 2018, 272, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Gao, F.; Yu, C.; Zeng, M.; He, G.; Wu, Y.; Su, Y.; Hu, N.; Zhou, Z.; Yang, Z.; et al. Dual-targeted therapy in HER2-positive breast cancer cells with the combination of carbon dots/HER3 siRNA and trastuzumab. Nanotechnology 2020, 31, 335102. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Brighi, C.; Reid, L.; White, A.L.; Genovesi, L.A.; Kojic, M.; Millar, A.; Bruce, Z.; Day, B.W.; Rose, S.; Whittaker, A.K.; et al. MR-guided focused ultrasound increases antibody delivery to nonenhancing high-grade glioma. Neurooncol. Adv. 2020, 2, vdaa030. [Google Scholar] [CrossRef] [Green Version]
- Kubo, S.; Takagi-Kimura, M.; Tagawa, M.; Kasahara, N. Dual-vector prodrug activator gene therapy using retroviral replicating vectors. Cancer Gene. Ther. 2019, 26, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Moro, R.; Tcherkassova, J.; Song, E. A New Broad-Spectrum Cancer Marker. IVD Technology. 2005. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.605.495&rep=rep1&type=pdf (accessed on 9 March 2022).
- Moskaleva, E.Y.; Posypanova, G.A.; Koromyslova, I.A.; Shmyrev, I.I.; Krivonos, A.V.; Myagkikh, I.V.; Feldman, N.B.; Finakova, G.V.; Katukov, V.Y.; Luzhkov, Y.M.; et al. In vivo antitumor activity of cytotoxic drugs conjugated with human alpha-fetoprotein. Tumor Target. 1996, 2, 299–306. [Google Scholar]
- Yabbarov, N.G.; Mollaev, M.D.; Zabolotskii, A.I.; Mazalev, D.A.; Gorokhovets, N.V.; Sokol, M.B.; Mollaeva, M.R.; Fomicheva, M.V.; Pshenichnikova, A.B.; Nikolskaya, E.D. Obtaining and Purification of Recombinant Domain III of Human Alpha-Fetoprotein. Biotekhnologiya 2021, 37, 32–42. [Google Scholar] [CrossRef]
- Mollaev, M.; Zabolotskii, A.; Gorokhovets, N.; Nikolskaya, E.; Sokol, M.; Tsedilin, A.; Mollaeva, M.; Chirkina, M.; Kuvaev, T.; Pshenichnikova, A.; et al. Expression of acid cleavable Asp-Pro linked multimeric AFP peptide in E. coli. J. Genet. Eng. Biotechnol. 2021, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Severin, S.E.; Posypanova, G.A.; Katukov, V.Y.; Shmyrev, I.I.; Luzhkov, Y.M.; Gerasimova, G.K.; Zhukova, O.S.; Vorozhtsov, G.N.; Kaliya, O.L.; Lukyanets, E.A.; et al. Antitumor activity of conjugates of the oncofetal protein alpha-fetoprotein and phthalocyanines in vitro. Biochem. Mol. Biol. Int. 1997, 43, 1081–1089. [Google Scholar]
- Posypanova, G.A.; Gorokhovets, N.V.; Makarov, V.A.; Savvateeva, L.V.; Kireeva, N.N.; Severin, S.E.; Severin, E.S. Recombinant alpha-fetoprotein C-terminal fragment: The new recombinant vector for targeted delivery. J. Drug Target. 2008, 16, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Godovanny, A.V.; Savvateeva, M.V.; Sotnichenko, A.I.; Yabbarov, N.G.; Klimova, O.V.; Gnuchev, N.V.; Severin, S.E. In vitro antitumor activity studying of AFP recombinat C-ending domain conjugate with cisplatine. Mol. Med. 2011, 1, 44–48. [Google Scholar]
- Zhang, J.; Alcaide, P.; Liu, L.; Sun, J.; He, A.; Luscinskas, F.W.; Shi, G.P. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS ONE 2011, 6, e14525. [Google Scholar] [CrossRef] [PubMed]
- Eichbaum, C.; Meyer, A.S.; Wang, N.; Bischofs, E.; Steinborn, A.; Bruckner, T.; Brodt, P.; Sohn, C.; Eichbaum, M.H. Breast cancer cell-derived cytokines, macrophages and cell adhesion: Implications for metastasis. Anticancer Res. 2011, 31, 3219–3227. [Google Scholar] [PubMed]
- Blackwell, J.E.; Dagia, N.M.; Dickerson, J.B.; Berg, E.L.; Goetz, D.J. Ligand coated nanosphere adhesion to E- and P-selectin under static and flow conditions. Ann. Biomed. Eng. 2001, 29, 523–533. [Google Scholar] [CrossRef]
- Dickerson, J.B.; Blackwell, J.E.; Ou, J.J.; Shinde Patil, V.R.; Goetz, D.J. Limited adhesion of biodegradable microspheres to E- and P-selectin under flow. Biotechnol. Bioeng. 2001, 73, 500–509. [Google Scholar] [CrossRef]
- Kessner, S.; Krause, A.; Rothe, U.; Bendas, G. Investigation of the cellular uptake of E-Selectin-targeted immunoliposomes by activated human endothelial cells. Biochim. Biophys. Acta 2001, 1514, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Natoni, A.; Macauley, M.S.; O’Dwyer, M.E. Targeting Selectins and Their Ligands in Cancer. Front. Oncol. 2016, 6, 93. [Google Scholar] [CrossRef] [Green Version]
- Chantarasrivong, C.; Ueki, A.; Ohyama, R.; Unga, J.; Nakamura, S.; Nakanishi, I.; Higuchi, Y.; Kawakami, S.; Ando, H.; Imamura, A.; et al. Synthesis and Functional Characterization of Novel Sialyl LewisX Mimic-Decorated Liposomes for E-selectin-Mediated Targeting to Inflamed Endothelial Cells. Mol. Pharm. 2017, 14, 1528–1537. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, S.; Liu, Y.; Wang, S.; Zhang, J.; Huang, R. A Peptide Analogue of Selectin Ligands Attenuated Atherosclerosis by Inhibiting Monocyte Activation. Mediat. Inflamm. 2019, 2019, 8709583. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, S.; Sugihara, K.; Shibata, T.K.; Nakayama, J.; Akama, T.O.; Tamura, N.; Wong, S.M.; Bobkov, A.A.; Takano, Y.; Ohyama, C.; et al. Targeted drug delivery to tumor vasculature by a carbohydrate mimetic peptide. Proc. Natl. Acad. Sci. USA 2011, 108, 19587–19592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pippin, C.G.; Parker, T.A.; McMurry, T.J.; Brechbiel, M.W. Spectrophotometric method for the determination of a bifunctional DTPA ligand in DTPA-monoclonal antibody conjugates. Bioconjug. Chem. 1992, 3, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Gouin, S.; Winnik, F.M. Quantitative assays of the amount of diethylenetriaminepentaacetic acid conjugated to water-soluble polymers using isothermal titration calorimetry and colorimetry. Bioconjug. Chem. 2001, 12, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Guzmán, J.; Jiménez, V.A.; Alderete, J.B. Partially PEGylated PAMAM dendrimers as solubility enhancers of Silybin. Pharm. Dev. Technol. 2018, 23, 689–696. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Wang, Y.; Xu, C.; Dong, C.; Li, C.; Ren, S.; Zhang, W.; Lu, Y.; Dai, Y.; et al. Pharmacokinetics study of hemin in rats by applying 58Fe-extrinsically labeling techniques in combination with ICP-MS method. J. Pharm. Biomed. Anal. 2014, 88, 331–336. [Google Scholar] [CrossRef]
- Pichler, V.; Mayr, J.; Heffeter, P.; Dömötör, O.; Enyedy, É.A.; Hermann, G.; Groza, D.; Köllenspeger, G.; Galanksi, M.; Berger, W.; et al. Maleimide-functionalised platinum(IV) complexes as a synthetic platform for targeted drug de-livery. Chem. Commun. 2013, 49, 2249–2251. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; Patri, A.K.; Simak, J.; Hall, J.B.; Semberova, J.; De Paoli Lacerda, S.H.; McNeil, S.E. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol. Pharm. 2012, 9, 382–393. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, 469–471. [Google Scholar] [CrossRef]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.J. Comparison of magnetic properties of MRI contrast media solutions a different magnetic field strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to Image J: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Dąbkowska, M.; Łuczkowska, K.; Rogińska, D.; Sobuś, A.; Wasilewska, M.; Ulańczyk, Z.; Machaliński, B. Novel design of (PEG-ylated)PAMAM-based nanoparticles for sustained delivery of BDNF to neurotoxin-injured differentiated neuroblastoma cells. J. Nanobiotechnol. 2020, 18, 120. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yabbarov, N.; Nikolskaya, E.; Sokol, M.; Mollaeva, M.; Chirkina, M.; Seregina, I.; Gulyaev, M.; Pirogov, Y.; Petrov, R. Synergetic Enhancement of Tumor Double-Targeted MRI Nano-Probe. Int. J. Mol. Sci. 2022, 23, 3119. https://doi.org/10.3390/ijms23063119
Yabbarov N, Nikolskaya E, Sokol M, Mollaeva M, Chirkina M, Seregina I, Gulyaev M, Pirogov Y, Petrov R. Synergetic Enhancement of Tumor Double-Targeted MRI Nano-Probe. International Journal of Molecular Sciences. 2022; 23(6):3119. https://doi.org/10.3390/ijms23063119
Chicago/Turabian StyleYabbarov, Nikita, Elena Nikolskaya, Maria Sokol, Mariia Mollaeva, Margarita Chirkina, Irina Seregina, Mikhail Gulyaev, Yury Pirogov, and Rem Petrov. 2022. "Synergetic Enhancement of Tumor Double-Targeted MRI Nano-Probe" International Journal of Molecular Sciences 23, no. 6: 3119. https://doi.org/10.3390/ijms23063119