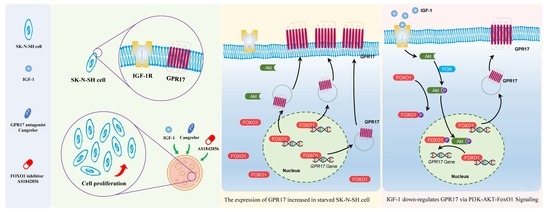
Insulin-like Growth Factor 1 Promotes Cell Proliferation by Downregulation of G-Protein-Coupled Receptor 17 Expression via PI3K/Akt/FoxO1 Signaling in SK-N-SH Cells
Abstract
:1. Introduction
2. Results
2.1. IGF-1 Downregulates FoxO1 and GPR17 Expression in SK-N-SH Cells
2.2. Blocking FOXO1 and GPR17 Promotes SK-N-SH Cell Proliferation
2.3. FoxO1 Regulates GPR17 Expression
2.4. IGF-1 Influences the PI3K/Akt/FoxO1 Signaling Cascade and Thus GPR17 Expression in SK-N-SH Cells
2.5. IGF-1 Promotes FoxO1 Nuclear Export and Reduces the Binding of FoxO1 to the GPR17 Promoter in SK-N-SH Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Measurement of Cell Viability
4.3. Cell Proliferation
4.4. Construction of Lentivirus-Mediated Short Hairpin RNA (shRNA)
4.5. Construction of Adenoviral Vectors
4.6. RT-qPCR
4.7. Western Blot Analysis
4.8. Immunofluorescence Cell Staining
4.9. Chromatin Immunoprecipitation qPCR
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Acute ischemic stroke |
| CHIP | Chromatin immunoprecipitation |
| EdU | 5-Ethynyl-2-deoxyuridine |
| EdU+, EdU- | Positive staining |
| FoxO1 | Forkhead box protein O1 (protein) |
| FOXO1 | Forkhead box protein O1 (gene) |
| GPR17 | G-protein-coupled receptor 17 |
| IGF-1 | Insulin-like growth factor 1 |
| qPCR | Real-time polymerase chain reaction |
| RT-qPCR | Quantitative reverse-transcription PCR |
| shRNA | Short hairpin RNA |
| WB | Western blot |
References
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA 2021, 325, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J. Acute Ischemic Stroke. N. Engl. J. Med. 2020, 383, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Phipps, M.S.; Cronin, C.A. Management of acute ischemic stroke. BMJ 2020, 368, l6983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennuto, M.; Pandey, U.B.; Polanco, M.J. Insulin-like growth factor 1 signaling in motor neuron and polyglutamine diseases: From molecular pathogenesis to therapeutic perspectives. Front. Neuroendocrinol. 2020, 57, 100821. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wong, H.S.C.; Wu, C.C.; Chiang, Y.H.; Chiu, W.T.; Chen, K.Y.; Chang, W.C. The functional roles of IGF-1 variants in the susceptibility and clinical outcomes of mild traumatic brain injury. J. Biomed. Sci. 2019, 26, 94. [Google Scholar] [CrossRef] [Green Version]
- Mangiola, A.; Vigo, V.; Anile, C.; De Bonis, P.; Marziali, G.; Lofrese, G. Role and Importance of IGF-1 in Traumatic Brain Injuries. Biomed Res. Int. 2015, 2015, 736104. [Google Scholar] [CrossRef] [Green Version]
- Mangiola, A.; Vigo, V.; Anile, C.; De Bonis, P.; Marziali, G.; Lofrese, G. Insulin-like growth factor-1 as a vascular protective factor. Circulation 2004, 110, 2260–2265. [Google Scholar]
- Dudek, H.; Datta, S.R.; Franke, T.F.; Birnbaum, M.J.; Yao, R.; Cooper, G.M.; Segal, R.A.; Kaplan, D.R.; Greenberg, M.E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 1997, 275, 661–665. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Hu, Z.; Ling, S.; Fang, M. Neuroprotective effects of DAHP and Triptolide in focal cerebral ischemia via apoptosis inhibition and PI3K/Akt/mTOR pathway activation. Front. Neuroanat. 2015, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, E.E.; Theibert, A.B. Functions of PI 3-kinase in development of the nervous system. Int. J. Dev. Neurosci. 2002, 20, 187–197. [Google Scholar] [CrossRef]
- Ma, N.; Zhao, Z.A.; Zhang, N.N.; Chen, H.S. Intra-arterial human urinary kallidinogenase alleviates brain injury in rats with permanent middle cerebral artery occlusion through PI3K/AKT/FoxO1 signaling pathway. Brain Res. 2018, 1687, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ciana, P.; Fumagalli, M.; Trincavelli, M.L.; Verderio, C.; Rosa, P.; Lecca, D.; Ferrario, S.; Parravicini, C.; Capra, V.; Gelosa, P.; et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006, 25, 4615–4627. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.; Parravicini, C.; Lecca, D.; Zanier, E.R.; Heine, C.; Bremicker, K.; Fumagalli, M.; Rosa, P.; Longhi, L.; Stocchetti, N.; et al. Changes of the GPR17 receptor, a new target for neurorepair, in neurons and glial cells in patients with traumatic brain injury. Purinergic Signal. 2013, 9, 451–462. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Wang, H.; Li, C.X.; Song, S.W.; Fang, S.H.; Wei, E.Q.; Shi, Q.J. GPR17 mediates ischemia-like neuronal injury via microglial activation. Int. J. Mol. Med. 2018, 42, 2750–2762. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Zhao, C.Z.; Zhang, X.Y.; Huang, X.Q.; Shi, W.Z.; Fang, S.H.; Lu, Y.B.; Zhang, W.P.; Xia, Q.; Wei, E.Q. The new P2Y-like receptor G protein-coupled receptor 17 mediates acute neuronal injury and late microgliosis after focal cerebral ischemia in rats. Neuroscience 2012, 202, 42–57. [Google Scholar] [CrossRef]
- Alavi, M.S.; Karimi, G.; Roohbakhsh, A. The role of orphan G protein-coupled receptors in the pathophysiology of multiple sclerosis: A review. Life Sci. 2019, 224, 33–40. [Google Scholar] [CrossRef]
- Fumagalli, M.; Lecca, D.; Abbracchio, M.P. CNS remyelination as a novel reparative approach to neurodegenerative diseases: The roles of purinergic signaling and the P2Y-like receptor GPR17. Neuropharmacology 2016, 104, 82–93. [Google Scholar] [CrossRef]
- Ceruti, S.; Villa, G.; Genovese, T.; Mazzon, E.; Longhi, R.; Rosa, P.; Bramanti, P.; Cuzzocrea, S.; Abbracchio, M.P. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain A J. Neurol. 2009, 132, 2206–2218. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Orozco, I.J.; Su, Y.; Suyama, S.; Gutiérrez-Juárez, R.; Horvath, T.L.; Wardlaw, S.L.; Plum, L.; Arancio, O.; Accili, D. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell 2012, 149, 1314–1326. [Google Scholar] [CrossRef] [Green Version]
- Mastaitis, J.; Min, S.; Elvert, R.; Kannt, A.; Xin, Y.; Ochoa, F.; Gale, N.W.; Valenzuela, D.M.; Murphy, A.J.; Yancopoulos, G.D.; et al. GPR17 gene disruption does not alter food intake or glucose homeostasis in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 1845–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Cook, J.R.; Kon, N.; Accili, D. Gpr17 in AgRP Neurons Regulates Feeding and Sensitivity to Insulin and Leptin. Diabetes 2015, 64, 3670–3679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marucci, G.; Dal Ben, D.; Lambertucci, C.; Martí Navia, A.; Spinaci, A.; Volpini, R.; Buccioni, M. GPR17 receptor modulators and their therapeutic implications: Review of recent patents. Expert Opin. Ther. Pat. 2019, 29, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, E.; Bonifacino, T.; Raffaele, S.; Milanese, M.; Morgante, E.; Bonanno, G.; Abbracchio, M.P.; Fumagalli, M. Abnormal Upregulation of GPR17 Receptor Contributes to Oligodendrocyte Dysfunction in SOD1 G93A Mice. Int. J. Mol. Sci. 2020, 21, 2395. [Google Scholar] [CrossRef] [Green Version]
- Lecca, D.; Raffaele, S.; Abbracchio, M.P.; Fumagalli, M. Regulation and signaling of the GPR17 receptor in oligodendroglial cells. Glia 2020, 68, 1957–1967. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, P.; Pan, Z.; Liu, D.; Niu, Y.; Zhu, W.; Yao, P.; Song, Y.; Sun, Y. Combination Therapy with Hyperbaric Oxygen and Erythropoietin Inhibits Neuronal Apoptosis and Improves Recovery in Rats with Spinal Cord Injury. Phys. Ther. 2019, 99, 1679–1689. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, P.; Pan, Z.; Liu, D.; Niu, Y.; Zhu, W.; Yao, P.; Song, Y.; Sun, Y. Neuroprotective effects of phytosterols and flavonoids from Cirsium setidens and Aster scaber in human brain neuroblastoma SK-N-SH cells. Life Sci. 2016, 148, 173–182. [Google Scholar]
- Kongsuphol, P.; Mukda, S.; Nopparat, C.; Villarroel, A.; Govitrapong, P. Melatonin attenuates methamphetamine-induced deactivation of the mammalian target of rapamycin signaling to induce autophagy in SK-N-SH cells. J. Pineal Res. 2009, 46, 199–206. [Google Scholar] [CrossRef]
- Kim, K.Y.; Cho, H.S.; Lee, S.H.; Ahn, J.H.; Cheon, H.G. Neuroprotective effects of KR-62980, a new PPARγ agonist, against chemical ischemia-reperfusion in SK-N-SH cells. Brain Res. 2011, 1372, 103–114. [Google Scholar] [CrossRef]
- Costales, J.; Kolevzon, A. The therapeutic potential of insulin-like growth factor-1 in central nervous system disorders. Neurosci. Biobehav. Rev. 2016, 63, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-Like Growth Factor-1 and Neuroinflammation. Front. Aging Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef]
- Zappelli, E.; Daniele, S.; Abbracchio, M.P.; Martini, C.; Trincavelli, M.L. A rapid and efficient immunoenzymatic assay to detect receptor protein interactions: G protein-coupled receptors. Int. J. Mol. Sci. 2014, 15, 6252–6264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, N.; Novatchkova, M.; Bachmair, A. Cellular Control of Protein Turnover via the Modification of the Amino Terminus. Int. J. Mol. Sci. 2021, 22, 3545. [Google Scholar] [CrossRef]
- Deng, S.; Jin, P.; Sherchan, P.; Liu, S.; Cui, Y.; Huang, L.; Zhang, J.H.; Gong, Y.; Tang, J. Recombinant CCL17-dependent CCR4 activation alleviates neuroinflammation and neuronal apoptosis through the PI3K/AKT/Foxo1 signaling pathway after ICH in mice. J. Neuroinflamm. 2021, 18, 62. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Zhong, J.; Shao, M.; Jiang, J.; Song, J.; Zhu, W.; Zhang, F.; Xu, H.; Xu, G.; et al. CD73 alleviates GSDMD-mediated microglia pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling. Clin. Transl. Med. 2021, 11, e269. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Luo, H.; Huang, K.; Guo, H.; Qu, Y.; Zhu, X. CRNDE/ETS1/GPR17 Facilitates the Proliferation, Migration, and Invasion of Glioma. Comput. Math. Methods Med. 2021, 2021, 7566365. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Coppi, E.; Maraula, G.; Fumagalli, M.; Failli, P.; Cellai, L.; Bonfanti, E.; Mazzoni, L.; Coppini, R.; Abbracchio, M.P.; Pedata, F.; et al. UDP-glucose enhances outward K(+) currents necessary for cell differentiation and stimulates cell migration by activating the GPR17 receptor in oligodendrocyte precursors. Glia 2013, 61, 1155–1171. [Google Scholar] [CrossRef]
- Boccazzi, M.; Lecca, D.; Marangon, D.; Guagnini, F.; Abbracchio, M.P.; Ceruti, S. A new role for the P2Y-like GPR17 receptor in the modulation of multipotency of oligodendrocyte precursor cells in vitro. Purinergic Signal. 2016, 12, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Liu, R.; Liu, G.; Hang, M.; Chen, G.; Xu, L.; Jin, Q.; Guo, D.; Kang, Q. Cangrelor ameliorates CLP-induced pulmonary injury in sepsis by inhibiting GPR17. Eur. J. Med. Res. 2021, 26, 70. [Google Scholar] [CrossRef]
- Zhan, T.W.; Tian, Y.X.; Wang, Q.; Wu, Z.X.; Zhang, W.P.; Lu, Y.B.; Wu, M. Cangrelor alleviates pulmonary fibrosis by inhibiting GPR17-mediated inflammation in mice. Int. Immunopharmacol. 2018, 62, 261–269. [Google Scholar] [CrossRef]









| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| FOXO1 | TGTCCTACGCCGACCTCATCAC | GCACGCTCTTGACCATCCACTC |
| GPR17 | GTTGGCAATACCCTGGCTCTGTG | GGACCAGCACGCACGACAAG |
| β-actin | TGGACTTCGAGCAAGAGATG | GAAGGAAGGCTGGAAGAGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-N.; Zhang, K.; Zhao, W.; Huang, S.-Y.; Li, H. Insulin-like Growth Factor 1 Promotes Cell Proliferation by Downregulation of G-Protein-Coupled Receptor 17 Expression via PI3K/Akt/FoxO1 Signaling in SK-N-SH Cells. Int. J. Mol. Sci. 2022, 23, 1513. https://doi.org/10.3390/ijms23031513
Lin K-N, Zhang K, Zhao W, Huang S-Y, Li H. Insulin-like Growth Factor 1 Promotes Cell Proliferation by Downregulation of G-Protein-Coupled Receptor 17 Expression via PI3K/Akt/FoxO1 Signaling in SK-N-SH Cells. International Journal of Molecular Sciences. 2022; 23(3):1513. https://doi.org/10.3390/ijms23031513
Chicago/Turabian StyleLin, Ka-Na, Kan Zhang, Wei Zhao, Shi-Ying Huang, and Hao Li. 2022. "Insulin-like Growth Factor 1 Promotes Cell Proliferation by Downregulation of G-Protein-Coupled Receptor 17 Expression via PI3K/Akt/FoxO1 Signaling in SK-N-SH Cells" International Journal of Molecular Sciences 23, no. 3: 1513. https://doi.org/10.3390/ijms23031513