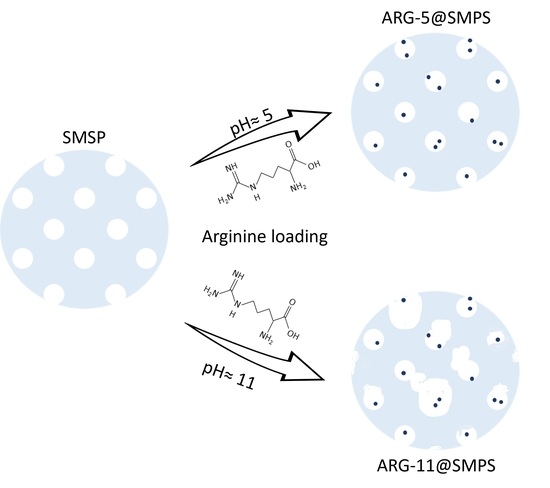
The Role of the pH in the Impregnation of Spherical Mesoporous Silica Particles with L-Arginine Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of SMSP
2.2. Characterization of ARG-11@SMSP
2.3. Characterization of ARG-5@SMSP and ARG-9@SMSP
3. Materials and Methods
3.1. Materials
3.2. The Synthesis of SMSP
3.3. Loading and Desorption of ARG
3.4. Instrumental Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, M.; Stievano, L.; Lambert, J.F. Adsorption and thermal condensation mechanisms of amino acids on oxide supports. 1. Glycine on silica. Langmuir 2004, 20, 914–923. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, W.; Xu, Y.; Wu, D.; Sun, Y.; Deng, F.; Shen, W. Amino acid adsorption on mesoporous materials: Influence of types of amino acids, modification of mesoporous materials, and solution conditions. J. Phys. Chem. B 2008, 112, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Goscianska, J.; Olejnik, A.; Pietrzak, R. Adsorption of l-phenylalanine onto mesoporous silica. Mater. Chem. Phys. 2013, 142, 586–593. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A.; Pietrzak, R. Comparison of ordered mesoporous materials sorption properties towards amino acids. Adsorption 2013, 19, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Gad, M.Z. Anti-aging effects of L-arginine. J. Adv. Res. 2010, 1, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K. Arginine. In Encyclopedia of Astrobiology; Springer: Berlin/Heidelberg, Germany, 2011; p. 85. ISBN 978-3-642-11274-4. [Google Scholar]
- Sudar-Milovanovic, E.; Obradovic, M.; Jovanovic, A.; Zaric, B.; Zafirovic, S.; Panic, A.; Radak, D.; Isenovic, E.R. Benefits of L-Arginine on Cardiovascular System. Rev. Med. Chem. 2016, 16, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; Freeman Publishing: New York, NY, USA, 2002. [Google Scholar]
- Vlasova, N.N.; Golovkova, L.P. The Adsorption of Amino Acids on the Surface of Highly Dispersed Silica. Colloid J. 2004, 66, 733–738. [Google Scholar] [CrossRef]
- Yazdani-Arazi, S.N.; Ghanbarzadeh, S.; Adibkia, K.; Kouhsoltani, M.; Hamishehkar, H. Histological evaluation of follicular delivery of arginine via nanostructured lipid carriers: A novel potential approach for the treatment of alopecia. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1379–1387. [Google Scholar] [CrossRef]
- Varedi, M.; Akbari, Z.; Dehghani, G.A.; Tabei, S.Z. Local Administration of L-Arginine Accelerates Wound Closure. Iran. J. Basic Med. Sci. 2009, 12, 173–178. [Google Scholar]
- McRae, M.P. Therapeutic Benefits of L-Arginine: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2016, 15, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Solanki, P.; Patel, A. In vitro release of l-arginine and cysteine from MCM-48: A study on effect of size of active biomolecules on release rate. J. Porous Mater. 2018, 25, 1489–1498. [Google Scholar] [CrossRef]
- Reesi, F.; Minaiyan, M.; Taheri, A. A novel lignin-based nanofibrous dressing containing arginine for wound-healing applications. Drug Deliv. Transl. Res. 2018, 8, 111–122. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, A.J.; Hokura, A.; Kisler, J.M.; Shimazu, S.; Stevens, G.W.; Komatsu, Y. Amino acid adsorption onto mesoporous silica molecular sieves. Sep. Purif. Technol. 2006, 48, 197–201. [Google Scholar] [CrossRef]
- Vinu, A.; Hossain, K.Z.; Satish Kumar, G.; Sivamurugan, V.; Ariga, K. Adsorption of amino acid on mesoporous molecular sieves. Nanoporous Mater. IV 2005, 156, 631–636. [Google Scholar]
- Vinu, A.; Hossain, K.Z.; Satish Kumar, G.; Ariga, K. Adsorption of l-histidine over mesoporous carbon molecular sieves. Carbon N. Y. 2006, 44, 530–536. [Google Scholar] [CrossRef]
- Landau, M.V.; Varkey, S.P.; Herskowitz, M.; Regev, O.; Pevzner, S.; Sen, T.; Luz, Z. Wetting stability of Si-MCM-41 mesoporous material in neutral, acidic and basic aqueous solutions. Microporous Mesoporous Mater. 1999, 33, 149–163. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Sansare, S.D. Thermal, hydrothermal and acid-base stability of highly siliceous MCM-41 mesoporous material. Proc. Indian Acad. Sci. Chem. Sci. 1997, 109, 229–233. [Google Scholar] [CrossRef]
- Ninni, L.; Meirelles, A.J.A. Water activity, ph and density of aqueous amino acids solutions. Biotechnol. Prog. 2001, 17, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Ambati, J.; Lopez, A.M.; Cochran, D.; Wattamwar, P.; Bean, K.; Dziubla, T.D.; Rankin, S.E. Engineered silica nanocarriers as a high-payload delivery vehicle for antioxidant enzymes. Acta Biomater. 2012, 8, 2096–2103. [Google Scholar] [CrossRef]
- Bass, J.D.; Grosso, D.; Boissiere, C.; Belamie, E.; Coradin, T.; Sanchez, C. Stability of mesoporous oxide and mixed metal oxide materials under biologically relevant conditions. Chem. Mater. 2007, 19, 4349–4356. [Google Scholar] [CrossRef]
- Chen, G.; Teng, Z.; Su, X.; Liu, Y.; Lu, G. Unique biological degradation behavior of stöber mesoporous silica nanoparticles from their interiors to their exteriors. J. Biomed. Nanotechnol. 2015, 11, 722–729. [Google Scholar] [CrossRef]
- He, Q.; Shi, J.; Zhu, M.; Chen, Y.; Chen, F. The three-stage in vitro degradation behavior of mesoporous silica in simulated body fluid. Microporous Mesoporous Mater. 2010, 131, 314–320. [Google Scholar] [CrossRef]
- Escax, V.; Delahaye, E.; Impéror-Clerc, M.; Beaunier, P.; Appay, M.D.; Davidson, A. Modifying the porosity of SBA-15 silicas by post-synthesis basic treatments. Microporous Mesoporous Mater. 2007, 102, 234–241. [Google Scholar] [CrossRef]
- Rimola, A.; Costa, D.; Sodupe, M.; Lambert, J.F.; Ugliengo, P. Silica Surface Features and Their Role in the Adsorption of Biomolecules: Computational Modeling and Experiments. Chem. Rev 2013, 113, 4216–4313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertl, W.; Hair, M.L. Hydrogen bonding between adsorbed gases and surface hydroxyl groups on silica. J. Phys. Chem. 1968, 72, 4676–4682. [Google Scholar] [CrossRef]
- Venyaminov, S.Y.; Kalnin, N.N. Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. I. Spectral parameters of amino acid residue absorption bands. Biopolymers 1990, 30, 1243–1257. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, M.; Hellwig, P. Infrared spectra and molar absorption coefficients of the 20 alpha amino acids in aqueous solutions in the spectral range from 1800 to 500 cm−1. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2006, 64, 987–1001. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 1954, 211, 907–913. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Rossi, S.; Bonferoni, M.C.; Riva, F.; Malavasi, L.; Caramella, C.; Ferrari, F. Freeze dried chitosan acetate dressings with glycosaminoglycans and traxenamic acid. Carbohydr. Polym. 2018, 184, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Mortera, R.; Fiorilli, S.; Garrone, E.; Verné, E.; Onida, B. Pores occlusion in MCM-41 spheres immersed in SBF and the effect on ibuprofen delivery kinetics: A quantitative model. Chem. Eng. J. 2010, 156, 184–192. [Google Scholar] [CrossRef]
- Gu, J.; Shi, J.; Xiong, L.; Chen, H.; Li, L.; Ruan, M. A new strategy to incorporate high density gold nanowires into the channels of mesoporous silica thin films by electroless deposition. Solid State Sci. 2004, 6, 747–752. [Google Scholar] [CrossRef]







| SSABET (m2/g) | Pore Volume (cm3/g) | |
|---|---|---|
| SMSP | 1143 | 0.82 |
| ARG-11@SMSP | 786 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, S.S.Y.; Martinez, S.; Banchero, M.; Manna, L.; Ronchetti, S.; Onida, B. The Role of the pH in the Impregnation of Spherical Mesoporous Silica Particles with L-Arginine Aqueous Solutions. Int. J. Mol. Sci. 2021, 22, 13403. https://doi.org/10.3390/ijms222413403
Mohamed SSY, Martinez S, Banchero M, Manna L, Ronchetti S, Onida B. The Role of the pH in the Impregnation of Spherical Mesoporous Silica Particles with L-Arginine Aqueous Solutions. International Journal of Molecular Sciences. 2021; 22(24):13403. https://doi.org/10.3390/ijms222413403
Chicago/Turabian StyleMohamed, Sara Saber Younes, Sonia Martinez, Mauro Banchero, Luigi Manna, Silvia Ronchetti, and Barbara Onida. 2021. "The Role of the pH in the Impregnation of Spherical Mesoporous Silica Particles with L-Arginine Aqueous Solutions" International Journal of Molecular Sciences 22, no. 24: 13403. https://doi.org/10.3390/ijms222413403