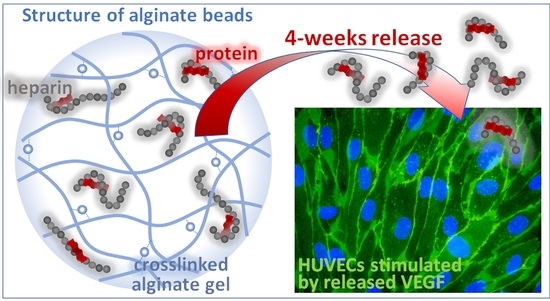
Complexation of CXCL12, FGF-2 and VEGF with Heparin Modulates the Protein Release from Alginate Microbeads
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Alg Microbeads
2.2. Interactions of Hep, HSA, and Proteins with Alg in the Solution and in the Form of Crosslinked Thin Layers
2.3. Release of CXCL12, FGF-2, and VEGF from Alg Microbeads



2.4. In Vitro Biological Evaluation
2.4.1. Effect of CXCL12 Released from Alg Microbeads on Migration of Jurkat Cells
2.4.2. Effect of FGF-2 and VEGF Released from Alg Microbeads on HUVEC Proliferation and Differentiation
Cell Adhesion and Proliferation

Cell Morphology and Expression of Specific Markers of Differentiation


3. Materials and Methods
3.1. Materials
3.2. Preparation of the Microbeads
| Sample Abbreviation | Alginate | Protein a | HSA | Heparin |
|---|---|---|---|---|
| (wt. %) | ||||
| Alg b | 1.3 | 0.003 | - | - |
| Alg/HSA b | 1.3 | 0.003 | 0.01 | - |
| Alg/Hep c | 1.3 | 0.003 | - | 0.03 |
| Alg/HSA/Hep b | 1.3 | 0.003 | 0.01 | 0.03 |
| Alg/HSA/Hep II b | 1.3 | 0.003 | 0.01 | 0.06 |
3.3. Characterization of the Microbeads
3.4. Evaluation of Interactions between Hep, HSA and Proteins with Alg Matrix
3.5. Protein Release Studies
3.6. In Vitro Studies
3.6.1. Bioactivity of CXCL12 Released from Alg Microbeads
3.6.2. Bioactivity of VEGF and FGF-2 Released from Alg Microbeads
| Experiment | Sample | Medium Abbreviation | Composition of the Cultivation Medium * |
|---|---|---|---|
| xCELLigence RTCA | Positive control | EGMfull (C+) | 2% of FS, EGF, Hep, ascorbic acid, hydrocortisone, 1% of ABAM, IGF, VEGF, FGF-2 (200 µL/well) |
| VEGF microbeads | EGMw | 2.2% of FS, EGF, Hep, ascorbic acid, hydrocortisone, 1% of ABAM (180 µL/well); 20 µL of the microbead eluate in PBS/well | |
| FGF-2 microbeads | EGMw | 2.2% of FS, EGF, Hep, ascorbic acid, hydrocortisone, 1% of ABAM (180 µL/well); 20 µL of the microbead eluate in PBS/well | |
| Negative control | EGMw (C-) | 2.2% of FS, EGF, Hep, ascorbic acid, hydrocortisone, 1% of ABAM (180 µL/well); 20 µL of PBS/well | |
| Immuno-fluorescence staining | Positive control | EGMfull (C+) | 2% of FS, Hep, ascorbic acid, hydrocortisone, 1% of ABAM, IGF, EGF, VEGF, FGF-2, (1000 µL/well) |
| VEGF microbeads | EGMm | 2.2% of FS, Hep, ascorbic acid, hydrocortisone, 1% of ABAM, IGF, EGF, 1/4 of the standard FGF-2 concentration (800 µL/well); 200 µL of the microbeads in EGMm/well | |
| Negative control (for VEGF) | EGMm (C-) | 2.2% of FS, Hep, ascorbic acid, hydrocortisone, 1% of ABAM, IGF, EGF, 1/4 of the standard FGF-2 concentration (1000 µL/well); | |
| FGF-2 microbeads | EGMm | 2.2% of FS, Hep, ascorbic acid, hydrocortisone, 1% of ABAM, IGF, EGF, 1/4 of the standard VEGF concentration (800 µL/well); 200 µL of the microbeads in EGMm/well | |
| Negative control (for FGF) | EGMm (C-) | 2.2% of FS, Hep, ascorbic acid, hydrocortisone, 1% of ABAM, IGF, EGF, 1/4 of the standard VEGF concentration (1000 µL/well); |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Aguilar, L.M.C.; Silva, S.M.; Moulton, S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Growth factor-delivery systems for tissue engineering: A materials perspective. Expert. Rev. Med. Devices 2006, 3, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 2012, 64, 194–205. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Wawrzyńska, E.; Kubies, D. Alginate matrices for protein delivery—A short review. Physiol. Res. 2018, 67 (Suppl. 2), S319–S334. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Huang, L.; Abdalla, A.M.E.; Xiao, L.; Yang, G. Biopolymer-Based Microcarriers for Three-Dimensional Cell Culture and Engineered Tissue Formation. Int. J. Mol. Sci. 2020, 21, 1895. [Google Scholar] [CrossRef] [Green Version]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumper, R.J.; Hoffman, A.S.; Puolakkainen, P.A.; Bouchard, L.S.; Gombotz, W.R. Calcium-alginate beads for the oral delivery of transrofming grosth faktro-beta(1) (TGF-beta(1))-stabilization of TGF-beta(1) by the addition of polyacrylic acid within acid-treated beads. J. Control. Release 1994, 30, 241–251. [Google Scholar] [CrossRef]
- Gu, F.; Amsden, B.; Neufeld, R. Sustained delivery of vascular endothelial growth factor with alginate beads. J. Control. Release 2004, 96, 463–472. [Google Scholar] [CrossRef]
- Ghiselli, G. Heparin Binding Proteins as Therapeutic Target: An Historical Account and Current Trends. Medicines 2019, 6, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudalla, G.A.; Murphy, W.L. Biomaterials that Regulate Growth Factor Activity via Bioinspired Interactions. Adv. Funct. Mater. 2011, 21, 1754–1768. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-based and heparin-inspired hydrogels: Size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef]
- Benoit, D.S.; Collins, S.D.; Anseth, K.S. Multifunctional hydrogels that promote osteogenic hMSC differentiation through stimulation and sequestering of BMP2. Adv. Funct. Mater. 2007, 17, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.; Sabareeswaran, A.; Kumar, G.S.V. PEG grafted chitosan scaffold for dual growth factor delivery for enhanced wound healing. Sci. Rep. 2019, 9, 19165. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.-C.; Mi, F.-L.; Sung, H.-W.; Kuo, P.-L. Heparin-functionalized chitosan–alginate scaffolds for controlled release of growth factor. Int. J. Pharm. 2009, 376, 69–75. [Google Scholar] [CrossRef]
- Jha, A.K.; Mathur, A.; Svedlund, F.L.; Ye, J.; Yeghiazarians, Y.; Healy, K.E. Molecular weight and concentration of heparin in hyaluronic acid-based matrices modulates growth factor retention kinetics and stem cell fate. J. Control. Release 2015, 209, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Jeon, O.; Powell, C.; Solorio, L.D.; Krebs, M.D.; Alsberg, E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J. Control. Release 2011, 154, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Henderson, P.W.; Singh, S.P.; Krijgh, D.D.; Yamamoto, M.; Rafii, D.C.; Sung, J.J.; Rafii, S.; Rabbany, S.Y.; Spector, J.A. Stromal-derived factor-1 delivered via hydrogel drug-delivery vehicle accelerates wound healing in vivo. Wound Repair Regen. 2011, 19, 420–425. [Google Scholar] [CrossRef]
- Janssens, R.; Struyf, S.; Proost, P. The unique structural and functional features of CXCL12. Cell. Mol. Immunol. 2018, 15, 299–311. [Google Scholar] [CrossRef]
- Righetti, A.; Giulietti, M.; Šabanović, B.; Occhipinti, G.; Principato, G.; Piva, F. CXCL12 and Its Isoforms: Different Roles in Pancreatic Cancer? J. Oncol. 2019, 9681698. [Google Scholar] [CrossRef] [PubMed]
- García-Cuesta, E.M.; Santiago, C.A.; Vallejo-Díaz, J.; Juarranz, Y.; Rodríguez-Frade, J.M.; Mellado, M. The Role of the CXCL12/CXCR4/ACKR3 Axis in Autoimmune Diseases. Front. Endocrinol. 2019, 10, 585. [Google Scholar] [CrossRef] [Green Version]
- Guyon, A. CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front. Cell. Neurosci. 2014, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Alagpulinsa, D.A.; Cao, J.J.L.; Driscol, R.K.; Sirbulescu, R.F.; Penson, M.F.E.; Sremac, M.; Engquist, E.N.; Brauns, T.A.; Markmann, J.F.; Melton, D.A.; et al. Alginate-microencapsulation of human stem cell-derived beta cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am. J. Transplant. 2019, 19, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Sremac, M.; Lei, J.; Penson, M.F.E.; Schuetz, C.; Lakey, J.R.T.; Papas, K.K.; Varde, P.S.; Hering, B.; de Vos, P.; Brauns, T.; et al. Preliminary Studies of the Impact of CXCL12 on the Foreign Body Reaction to Pancreatic Islets Microencapsulated in Alginate in Nonhuman Primates. Transplant. Direct. 2019, 5, e447. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Yuan, J.; Duncanson, S.; Hibert, M.L.; Kodish, B.C.; Mylavaganam, G.; Maker, M.; Li, H.; Sremac, M.; Santosuosso, M.; et al. Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression. Am. J. Transplant. 2015, 15, 618–627. [Google Scholar] [CrossRef]
- Duncanson, S.; Sambanis, A. Dual factor delivery of CXCL12 and Exendin-4 for improved survival and function of encapsulated beta cells under hypoxic conditions. Biotechnol. Bioeng. 2013, 110, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Irvine, D.J. Engineering chemoattractant gradients using chemokine-releasing polysaccharide microspheres. Biomaterials 2011, 32, 4903–4913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, T.E.; Nunez, A.C.; Sunde, M.; Easterbrook-Smith, S.B. Serum albumin prevents protein aggregation and amyloid formation and retains chaperone-like activity in the presence of physiological ligands. J. Biol. Chem. 2012, 287, 21530–21540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mørch, Y.A.; Qi, M.; Gundersen, P.O.M.; Formo, K.; Lacik, I.; Skjåk-Bræk, G.; Oberholzer, J.; Strand, B.L. Binding and leakage of barium in alginate microbeads. J. Biomed. Mater. Res. A 2012, 100A, 2939–2947. [Google Scholar] [CrossRef] [Green Version]
- Qi, M.; Mørch, Y.; Lacík, I.; Formo, K.; Marchese, E.; Wang, Y.; Danielson, K.K.; Kinzer, K.; Wang, S.; Barbaro, B.; et al. Survival of human islets in microbeads containing high guluronic acid alginate crosslinked with Ca2+ and Ba2+. Xenotransplantation 2012, 19, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.M.; Loganathan, D.; Linhardt, R.J. Determination of the pKa of glucuronic acid and the carboxy groups of heparin by 13C-nuclear-magnetic-resonance spectroscopy. Biochem. J. 1991, 278, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Vilcacundo, R.; Mendez, P.; Reyes, W.; Romero, H.; Pinto, A.; Carrillo, W. Antibacterial Activity of Hen Egg White Lysozyme Denatured by Thermal and Chemical Treatments. Sci. Pharm. 2018, 86, 48. [Google Scholar] [CrossRef] [Green Version]
- Malamud, D.; Drysdale, J.W. Isoelectric points of proteins—Table. Anal. Biochem. 1978, 86, 620–647. [Google Scholar] [CrossRef]
- Xu, X.; Han, Q.; Shi, J.; Zhang, H.; Wang, Y. Structural, thermal and rheological characterization of bovine serum albumin binding with sodium alginate. J. Mol. Liq. 2020, 299, 112123. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Carvajal, M.T.; Harris, M.T. Interactions between bovine serum albumin and alginate: An evaluation of alginate as protein carrier. J. Colloid Interface Sci. 2009, 332, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Jay, S.M.; Saltzman, W.M. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J. Control. Release 2009, 134, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, T.X.; Rao, K.S.; Spees, J.L.; Oldinski, R.A. Osteogenic differentiation of human mesenchymal stem cells through alginate-graft-poly(ethylene glycol) microsphere-mediated intracellular growth factor delivery. J. Control. Release 2014, 192, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, E.; López-Noriega, A.; Thompson, E.M.; Hibbitts, A.; Cryan, S.A.; O'Brien, F.J. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen–hydroxyapatite scaffolds for promoting vascularization and bone repair. J. Tissue Eng. Regen. Med. 2017, 11, 1097–1109. [Google Scholar] [CrossRef]
- Campbell, K.T.; Hadley, D.J.; Kukis, D.L.; Silva, E.A. Alginate hydrogels allow for bioactive and sustained release of VEGF-C and VEGF-D for lymphangiogenic therapeutic applications. PLoS ONE 2017, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Peters, M.C.; Mooney, D.J. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J. Control. Release 2003, 87, 49–56. [Google Scholar] [CrossRef]
- Elçin, Y.M.; Dixit, V.; Gitnick, G. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: Implications for tissue engineering and wound healing. Artif. Organs 2001, 25, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Isenberg, B.C.; Rowley, J.A.; Mooney, D.J. Release from alginate enhances the biological activity of vascular endothelial growth factor. J. Biomater. Sci.-Polym. Ed. 1998, 9, 1267–1278. [Google Scholar] [CrossRef]
- Hao, X.; Silva, E.A.; Månsson-Broberg, A.; Grinnemo, K.H.; Siddiqui, A.J.; Dellgren, G.; Wärdell, E.; Brodin, L.A.; Mooney, D.J.; Sylvén, C. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc. Res. 2007, 75, 1781–1785. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.A.; Mooney, D.J. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 2010, 31, 1235–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, O.; Moya, M.L.; Opara, E.C.; Brey, E.M. Synthesis of multilayered alginate microcapsules for the sustained release of fibroblast growth factor-1. J. Biomed. Mater. Res. A 2010, 95, 632–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanihara, M.; Suzuki, Y.; Yamamoto, E.; Noguchi, A.; Mizushima, Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J. Biomed. Mater. Res. 2001, 56, 216–221. [Google Scholar] [CrossRef]
- Su, J.; Xu, H.; Sun, J.; Gong, X.; Zhao, H. Dual Delivery of BMP-2 and bFGF from a New Nano-Composite Scaffold, Loaded with Vascular Stents for Large-Size Mandibular Defect Regeneration. Int. J. Mol. Sci. 2013, 14, 12714–12728. [Google Scholar] [CrossRef] [Green Version]
- Miao, T.X.; Little, A.C.; Aronshtam, A.; Marquis, T.; Fenn, S.L.; Hristova, M.; Krementsov, D.N.; van der Vliet, A.; Spees, J.L.; Oldinski, R.A. Internalized FGF-2-Loaded Nanoparticles Increase Nuclear ERK1/2 Content and Result in Lung Cancer Cell Death. Nanomaterials 2020, 10, 612. [Google Scholar] [CrossRef] [Green Version]
- Greenwood-Goodwin, M.; Teasley, E.S.; Heilshorn, S.C. Dual-stage growth factor release within 3D protein-engineered hydrogel niches promotes adipogenesis. Biomater. Sci. 2014, 2, 1627–1639. [Google Scholar] [CrossRef]
- Wells, L.A.; Sheardown, H. Extended release of high pI proteins from alginate microspheres via a novel encapsulation technique. Eur. J. Pharm. Biopharm. 2007, 65, 329–335. [Google Scholar] [CrossRef]
- Rahmani, V.; Sheardown, H. Protein-alginate complexes as pH-/ion-sensitive carriers of proteins. Int. J. Pharm. 2018, 535, 452–461. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Zhu, Z. Study on the blends of silk fibroin and sodium alginate: Hydrogen bond formation, structure and properties. Polymer 2019, 163, 144–153. [Google Scholar] [CrossRef]
- Takacova, M.; Hlouskova, G.; Zatovicova, M.; Benej, M.; Sedlakova, O.; Kopacek, J.; Pastorek, J.; Lacik, I.; Pastorekova, S. Encapsulation of anti-carbonic anhydrase IX antibody in hydrogel microspheres for tumor targeting. J. Enzyme Inhib. Med. Chem. 2016, 31, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Stoppel, W.L.; White, J.C.; Horava, S.D.; Bhatia, S.R.; Roberts, S.C. Transport of biological molecules in surfactant-alginate composite hydrogels. Acta Biomater. 2011, 7, 3988–3998. [Google Scholar] [CrossRef] [Green Version]
- Venturoli, D.; Rippe, B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: Effects of molecular size, shape, charge, and deformability. Am. J. Physiol. Renal. Physiol. 2005, 288, F605–F613. [Google Scholar] [CrossRef] [PubMed]
- Ziarek, J.J.; Veldkamp, C.T.; Zhang, F.; Murray, N.J.; Kartz, G.A.; Liang, X.; Su, J.; Baker, J.E.; Linhardt, R.J.; Volkman, B.F. Heparin oligosaccharides inhibit chemokine (CXC motif) ligand 12 (CXCL12) cardioprotection by binding orthogonal to the dimerization interface, promoting oligomerization, and competing with the chemokine (CXC motif) receptor 4 (CXCR4) N terminus. J. Biol. Chem. 2013, 288, 737–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, I.; Kedem, A.; Cohen, S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials 2008, 29, 3260–3268. [Google Scholar] [CrossRef] [PubMed]
- Sedlář, A.; Trávníčková, M.; Matějka, R.; Pražák, Š.; Mészáros, Z.; Bojarová, P.; Bačáková, L.; Křen, V.; Slámová, K. Growth Factors VEGF-A165 and FGF-2 as Multifunctional Biomolecules Governing Cell Adhesion and Proliferation. Int. J. Mol. Sci. 2021, 22, 1843. [Google Scholar] [CrossRef]
- Forsten, K.E.; Fannon, M.; Nugent, M.A. Potential mechanisms for the regulation of growth factor binding by heparin. J. Theor. Biol. 2000, 205, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Majumdar, A.; Li, X.; Adler, J.; Sun, Z.; Vertuani, S.; Hellberg, C.; Mellberg, S.; Koch, S.; Dimberg, A.; et al. VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat. Commun. 2013, 4, 1672. [Google Scholar] [CrossRef] [Green Version]
- Tsuji-Tamura, K.; Ogawa, M. Inhibition of the PI3K–Akt and mTORC1 signaling pathways promotes the elongation of vascular endothelial cells. J. Cell Sci. 2016, 129, 1165–1178. [Google Scholar] [CrossRef] [Green Version]
- Chiaverina, G.; di Blasio, L.; Monica, V.; Accardo, M.; Palmiero, M.; Peracino, B.; Vara-Messler, M.; Puliafito, A.; Primo, L. Dynamic Interplay between Pericytes and Endothelial Cells during Sprouting Angiogenesis. Cells 2019, 8, 1109. [Google Scholar] [CrossRef] [Green Version]
- Filova, E.; Steinerova, M.; Travnickova, M.; Knitlova, J.; Musilkova, J.; Eckhardt, A.; Hadraba, D.; Matejka, R.; Prazak, S.; Stepanovska, J.; et al. Accelerated in vitro recellularization of decellularized porcine pericardium for cardiovascular grafts. Biomed. Mater. 2021, 16, 025024. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Kong, C.; Wen, S.; Shi, J. The use of heparin, bFGF, and VEGF 145 grafted acellular vascular scaffold in small diameter vascular graft. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 672–679. [Google Scholar] [CrossRef]
- Kasoju, N.; Pátíková, A.; Wawrzynska, E.; Vojtíšková, A.; Sedlačík, T.; Kumorek, M.; Pop-Georgievski, O.; Sticová, E.; Kříž, J.; Kubies, D. Bioengineering a pre-vascularized pouch for subsequent islet transplantation using VEGF-loaded polylactide capsules. Biomater. Sci. 2020, 8, 631–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gryshkov, O.; Mutsenko, V.; Tarusin, D.; Khayyat, D.; Naujok, O.; Riabchenko, E.; Nemirovska, Y.; Danilov, A.; Petrenko, A.Y.; Glasmacher, B. Coaxial Alginate Hydrogels: From Self-Assembled 3D Cellular Constructs to Long-Term Storage. Int. J. Mol. Sci. 2021, 22, 3096. [Google Scholar] [CrossRef]
- Sigal, G.B.; Mrksich, M.; Whitesides, G.M. Effect of surface wettability on the adsorption of proteins and detergents. J. Am. Chem. Soc. 1998, 120, 3464–3473. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Prashar, D.; Luk, Y.Y. Stereochemical effects of chiral monolayers on enhancing the resistance to mammalian cell adhesion. Chem. Commun. 2011, 47, 6165–6167. [Google Scholar] [CrossRef]
- Kumorek, M.; Kubies, D.; Riedel, T. Protein Interactions With Quaternized Chitosan/Heparin Multilayers. Physiol. Res. 2016, 65, S253–S261. [Google Scholar] [CrossRef] [PubMed]
- Pop-Georgievski, O.; Kubies, D.; Zemek, J.; Neykova, N.; Demianchuk, R.; Mazl Chanova, E.; Slouf, M.; Houska, M.; Rypacek, F. Self-assembled anchor layers/polysaccharide coatings on titanium surfaces: A study of functionalization and stability. Beilstein J. Nanotechnol. 2015, 6, 617–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adrian, E.; Treľová, D.; Filová, E.; Kumorek, M.; Lobaz, V.; Poreba, R.; Janoušková, O.; Pop-Georgievski, O.; Lacík, I.; Kubies, D. Complexation of CXCL12, FGF-2 and VEGF with Heparin Modulates the Protein Release from Alginate Microbeads. Int. J. Mol. Sci. 2021, 22, 11666. https://doi.org/10.3390/ijms222111666
Adrian E, Treľová D, Filová E, Kumorek M, Lobaz V, Poreba R, Janoušková O, Pop-Georgievski O, Lacík I, Kubies D. Complexation of CXCL12, FGF-2 and VEGF with Heparin Modulates the Protein Release from Alginate Microbeads. International Journal of Molecular Sciences. 2021; 22(21):11666. https://doi.org/10.3390/ijms222111666
Chicago/Turabian StyleAdrian, Edyta, Dušana Treľová, Elena Filová, Marta Kumorek, Volodymyr Lobaz, Rafal Poreba, Olga Janoušková, Ognen Pop-Georgievski, Igor Lacík, and Dana Kubies. 2021. "Complexation of CXCL12, FGF-2 and VEGF with Heparin Modulates the Protein Release from Alginate Microbeads" International Journal of Molecular Sciences 22, no. 21: 11666. https://doi.org/10.3390/ijms222111666