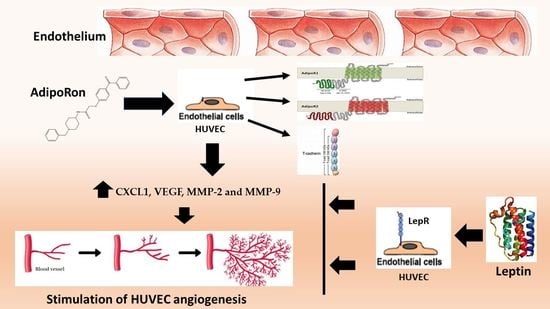
Adiponectin and Leptin Exert Antagonizing Effects on HUVEC Tube Formation and Migration Modulating the Expression of CXCL1, VEGF, MMP-2 and MMP-9
Abstract
:1. Introduction
2. Results
2.1. AdipoRon Reduces Cell Viability of HUVEC Endothelial Cells
2.2. AdipoRon and Leptin Exert Antagonizing Effects on HUVEC Tube Formation
2.3. AdipoRon and Leptin Exert Antagonizing Effects on HUVEC Cell Migration
2.4. AdipoRon and Leptin Exert Antagonizing Effects on HIF-1α, CXCL1, VEGF-A, MMP-2 and MMP-9 Expression in HUVEC Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. HUVEC Cell Culture
4.3. HUVEC Viability by XTT Assay
4.4. Wound Healing Assay
4.5. Tube Formation Assay on HUVEC
4.6. RNA Extraction and Quantitative Real Time-PCR (q-RT-PCR)
4.7. Western Blotting Assay
4.8. ELISA Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguilar-Ballester, M.; Hurtado-Genovés, G.; Taberner-Cortés, A.; Herrero-Cervera, A.; Martínez-Hervás, S.; González-Navarro, H. Therapies for the Treatment of Cardiovascular Disease Associated with Type 2 Diabetes and Dyslipidemia. Int. J. Mol. Sci. 2021, 22, 660. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2019, 10, 1568. [Google Scholar] [CrossRef] [Green Version]
- Aggoun, Y. Obesity, Metabolic Syndrome, and Cardiovascular Disease. Pediatr. Res. 2007, 61, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, H.; Xia, N. The Interplay between Adipose Tissue and Vasculature: Role of Oxidative Stress in Obesity. Front. Cardiovasc. Med. 2021, 8, 650214. [Google Scholar] [CrossRef] [PubMed]
- Adya, R.; Tan, B.K.; Randeva, H.S. Differential Effects of Leptin and Adiponectin in Endothelial Angiogenesis. J. Diabetes Res. 2015, 2015, 648239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrotta, F.; Nigro, E.; Mollica, M.; Costigliola, C.; D’Agnano, V.; Daniele, A.; Bianco, A.; Guerra, G. Pulmonary Hypertension and Obesity: Focus on Adiponectin. Int. J. Mol. Sci. 2019, 20, 912. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, R.; Fadaei, R.; Nahrkhalaji, A.S.; Panahi, G.; Fallah, S. The impacts of C1q/TNF-related protein-15 and adiponectin on Interleukin-6 and tumor necrosis factor-α in primary macrophages of patients with coronary artery diseases. Cytokine 2021, 142, 155470. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.E.; Scherer, P.E. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J. Intern. Med. 2005, 257, 167–175. [Google Scholar] [CrossRef]
- Okui, H.; Hamasaki, S.; Ishida, S.; Kataoka, T.; Orihara, K.; Fukudome, T.; Ogawa, M.; Oketani, O.; Saihara, K.; Shinsato, K.; et al. Adiponectin is a better predictor of endothelial function of the coronary artery than HOMA-R, body mass index, immunoreactive insulin, or triglycerides. Int. J. Cardiol. 2008, 126, 53–61. [Google Scholar] [CrossRef]
- Ouchi, N.; Kobayashi, H.; Kihara, S.; Kumada, M.; Sato, K.; Inoue, T.; Funahashi, T.; Walsh, K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2004, 279, 1304–1309. [Google Scholar] [CrossRef] [Green Version]
- Bråkenhielm, E.; Veitonmäki, N.; Cao, R.; Kihara, S.; Matsuzawa, Y.; Zhivotovsky, B.; Funahashi, T.; Cao, Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc. Natl. Acad. Sci. USA 2004, 101, 2476–2481. [Google Scholar] [CrossRef] [Green Version]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, Y.; Fan, Y.; Tang, Z.; Wang, N. Overexpression of Adiponectin Receptors Potentiates the Antiinflammatory Action of Subeffective Dose of Globular Adiponectin in Vascular Endothelial Cells. Arter. Thromb. Vasc. Biol. 2009, 29, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Parker-Duffen, J.L.; Nakamura, K.; Silver, M.; Kikuchi, R.; Tigges, U.; Yoshida, S.; Denzel, M.S.; Ranscht, B.; Walsh, K. T-cadherin Is Essential for Adiponectin-mediated Revascularization. J. Biol. Chem. 2013, 288, 24886–24897. [Google Scholar] [CrossRef] [Green Version]
- Joshi, M.B.; Philippova, M.; Ivanov, D.; Allenspach, R.; Erne, P.; Resink, T.J. T-cadherin protects endothelial cells from oxidative stress-induced apoptosis. FASEB J. 2005, 19, 1737–1739. [Google Scholar] [CrossRef]
- Rubina, K.A.; Kalinina, N.I.; Parfyonova, Y.V.; Tkachuk, V.A. T-cadherin as a receptor regulating angiogenesis and blood vessel remodeling. Biochem. Moscow. Suppl. 2007, 1, 57–63. [Google Scholar] [CrossRef]
- Margetic, S.; Gazzola, C.; Pegg, G.G.; Hill, R.A. Leptin: A review of its peripheral actions and interactions. Int. J. Obes. 2002, 11, 1407–1433. [Google Scholar] [CrossRef] [Green Version]
- Correia, M.L.G.; Rahmouni, K. Role of leptin in the cardiovascular and endocrine complications of metabolic syndrome. Diabetes, Obes. Metab. 2006, 6, 603–610. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Funahashi, T.; Kihara, S.; Shimomura, I. Adiponectin and Metabolic Syndrome. Thromb. Vasc. Biol. 2004, 24, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.N.; Chang, S.-D.; Peng, H.-H.; Lee, Y.-S.; Chang, Y.-L. Change in Amniotic Fluid Levels of Multiple Anti-Angiogenic Proteins before Development of Preeclampsia and Intrauterine Growth Restriction. J. Clin. Endocrinol. Metab. 2010, 95, 1431–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, A.M.; Andrade, S.; Pinho, F.; Monteiro, J.D.; Costa, M.; Lopes, C.; Aguas, A.P.; Monteiro, M.P. Prostate cancer cell proliferation and angiogenesis in different obese mice models. Int. J. Exp. Pathol. 2010, 91, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Magkos, F.; Brinkoetter, M.; Sienkiewicz, E.; Dardeno, T.A.; Kim, S.-Y.; Hamnvik, O.-P.R.; Koniaris, A. Leptin in human physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E567–E584. [Google Scholar] [CrossRef] [PubMed]
- Manuel-Apolinar, L.; López-Romero, R.; Zarate, A.; Damasio, L.; Ruiz, M.; Castillo-Hernández, C.; Guevara, G.; Mera-Jiménez, E. Leptin mediated ObRb receptor increases expression of adhesion intercellular molecules and cyclooxygenase 2 on murine aorta tissue inducing endothelial dysfunction. Int. J. Clin. Exp. Med. 2013, 6, 192–196. [Google Scholar] [PubMed]
- Cao, R.; Brakenhielm, E.; Wahlestedt, C.; Thyberg, J.; Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA 2001, 98, 6390–6395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-Y.; Kwon, H.M.; Lim, H.J.; Hong, B.K.; Lee, J.Y.; Park, B.E.; Jang, Y.S.; Cho, S.Y.; Kim, H.-S. Potential role of leptin in angiogenesis: Leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp. Mol. Med. 2001, 33, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Garonna, E.; Botham, K.M.; Birdsey, G.M.; Randi, A.M.; Gonzalez-Perez, R.R.; Wheeler-Jones, C.P.D. Vascular Endothelial Growth Factor Receptor-2 Couples Cyclo-Oxygenase-2 with Pro-Angiogenic Actions of Leptin on Human Endothelial Cells. PLoS ONE 2011, 6, e18823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribatti, D.; Nico, B.; Belloni, A.S.; Vacca, A.; Roncali, L.; Nussdorfer, G.G. Angiogenic activity of leptin in the chick embryo chorioallantoic membrane is in part mediated by endogenous fibroblast growth factor-2. Int. J. Mol. Med. 2001, 8, 265–268. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Han, B.; Wu, J.; Liu, D.-K.; Zou, J.-D.; Wang, M.; Liu, Z.-H. Effects of leptin-modified human placenta-derived mesenchymal stem cells on angiogenic potential and peripheral inflammation of human umbilical vein endothelial cells (HUVECs) after X-ray radiation. J. Zhejiang Univ. Sci. B 2020, 21, 327–340. [Google Scholar] [CrossRef]
- Guo, S.; Liu, M.; Wang, G.; Torroella-Kouri, M.; Gonzalez-Perez, R.R. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim. Biophys. Acta 2012, 1825, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Schroeter, M.R.; Schneiderman, J.; Schumann, B.; Glückermann, R.; Grimmas, P.; Buchwald, A.B.; Tirilomis, T.; Schöndube, F.A.; Konstantinides, S.V.; Schäfer, K. Expression of the leptin receptor in different types of vascular lesions. Histochem. Cell Biol. 2007, 128, 323–333. [Google Scholar] [CrossRef]
- Grabarek, B.O.; Kasela, T.; Adwent, I.; Zawidlak-Węgrzyńska, B.; Brus, R. Evaluation of the Influence of Adalimumab on the Expression Profile of Leptin-Related Genes and Proteins in Keratinocytes Treated with Lipopolysaccharide A. Int. J. Mol. Sci. 2021, 22, 1595. [Google Scholar] [CrossRef]
- Bishop, E.; Bell, G.T.; Bloor, S.; Broom, I.J.; Hendry, N.F.; Wheatley, D.N. An in vitro model of angiogenesis: Basic features. Angiogenesis 1999, 3, 335–344. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Di Somma, M.; Vliora, M.; Grillo, E.; Castro, B.; Dakou, E.; Schaafsma, W.; Vanparijs, J.; Corsini, M.; Ravelli, C.; Sakellariou, E.; et al. Role of VEGFs in metabolic disorders. Angiogenesis 2020, 23, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Delort, L.; Billard, H.; Vasson, M.-P.; Caldefie-Chezet, F. Breast cancer and obesity: In vitro interferences between adipokines and proangiogenic features and/or antitumor therapies? PLoS ONE 2013, 8, e58541. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, X.; Wang, R.; Sun, J.; Guo, B.; Wei, R.; Jia, Y. Adiponectin inhibits proliferation of vascular endothelial cells induced by Ox-LDL by promoting dephosphorylation of Caveolin-1 and depolymerization of eNOS and up-regulating release of NO. Int. Immunopharmacol. 2019, 73, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Bartolomé, M.V.; Miana, M.; Jurado-López, R.; Martín, R.; Zuluaga, P.; Martinez-Martinez, E.; Nieto, M.L.; Alvarez-Sala, L.A.; Millán, J.; et al. The Effects of Adiponectin and Leptin on Human Endothelial Cell Proliferation: A Live-Cell Study. J. Vasc. Res. 2012, 49, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; Maruyama, R.; Kawabata, Y.; Tajima, Y.; Takenaga, K. Antidiabetic adiponectin receptor agonist AdipoRon suppresses tumour growth of pancreatic cancer by inducing RIPK1/ERK-dependent necroptosis. Cell Death Dis. 2018, 9, 804. [Google Scholar] [CrossRef] [Green Version]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The Role of Adiponectin in Cancer: A Review of Current Evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Jiménez, F.; Pérez-Pérez, A.; de la Cruz-Merino, L.; Sánchez-Margalet, V. Obesity and Breast Cancer: Role of Leptin. Front Oncol. 2019, 9, 596. [Google Scholar] [CrossRef]
- Aronis, K.N.; Diakopoulos, N.; Fiorenza, C.G.; Chamberland, J.P.; Mantzoros, C.S. Leptin administered in physiological or pharmacological doses does not regulate circulatory angiogenic factors in human. Diabetologia 2011, 54, 2358–2367. [Google Scholar] [CrossRef] [Green Version]
- Mahadev, K.; Wu, X.; Donnelly, S.; Ouedraogo, R.; Eckhart, A.D.; Goldstein, B.J. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc. Res. 2008, 78, 376–384. [Google Scholar] [CrossRef]
- Wolk, R.; Deb, A.; Caplice, N.M.; Somers, V.K. Leptin receptor and functional effects of leptin in human endothelial progenitor cells. Atherosclerosis 2001, 183, 131–139. [Google Scholar] [CrossRef]
- Ouh, Y.-T.; Cho, H.W.; Lee, J.K.; Choi, S.H.; Choi, H.J.; Hong, J.H. CXC chemokine ligand 1 mediates adiponectin-induced angiogenesis in ovarian cancer. J. Mol. Endocrinol. 2017, 59, 285–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadson, K.; Chasiotis, H.; Wannaiampikul, S.; Tungtrongchitr, R.; Xu, A.; Sweeney, G. Adiponectin mediated APPL1-AMPK signaling induces cell migration, MMP activation, and collagen remodeling in cardiac fibroblasts. J. Cell Biochem. 2014, 115, 785–793. [Google Scholar] [CrossRef]
- Lee, Y.-A.; Ji, H.-I.; Lee, S.-H.; Hong, S.-J.; Yang, H.I.; Yoo, M.C.; Kim, K.S. The role of adiponectin in the production of IL-6, IL-8, VEGF and MMPs in human endothelial cells and osteoblasts: Implications for arthritic joints. Exp. Mol. Med. 2014, 46, e72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigro, E.; Orlandella, F.M.; Polito, R.; Mariniello, R.M.; Monaco, M.L.; Mallardo, M.; De Stefano, A.E.; Iervolino, P.L.C.; Salvatore, G.; Daniele, A. Adiponectin and leptin exert antagonizing effects on proliferation and motility of papillary thyroid cancer cell lines. J. Physiol. Biochem. 2021, 77, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Takahashia, E.; Saruwatarib, J.; Taniharac, H.; Inoue, T. The angiogenic effects of exosomes secreted from retinal pigment epithelial cells on endothelial cells. Biochem. Biophys. Rep. 2020, 22, 100760. [Google Scholar]





| Gene | Primer Sequence | Melting Temperature (°C) |
|---|---|---|
| GAPDH fw | 5′-CAT GGC CTT CCG TGT TCC TA-3′ | 59.3 °C |
| GAPDH rev | 5′-CCT GCT TCA CCA CCT TCT TGA T-3′ | 60.3 °C |
| MMP-2 fw | 5′-TGGCAAGTACGGCTTCTGTC-3′ | 59.3 °C |
| MMP-2 rev | 5′-TTCTTGTCGCGGTCGTAGTC-3′ | 60.3 °C |
| MMP-9 fw | 5′-TGCGCTACCACCTCGAACTT-3′ | 59.3 °C |
| MMP-9 rev | 5′-GATGCCATTGACGTCGTCCT-3′ | 75.6 °C |
| VEGF-A fw | 5′-CGGCGAAGAGAAGAGACACA-3′ | 59.3 °C |
| VEGF-A rev | 5′-GGAGGAAGGTC- AACCACTCA-3′ | 59.3 °C |
| CXCL1 fw | 5′-GCGCCCAAACCGAAGTCATA-3′ | 59.3 °C |
| CXCL1 rev | 5′-ATGGGGGATGCAGGATTGAG-3′ | 59.3 °C |
| HIF-1A fw | 5′- GAAAGCGCAAGTCTTCAAAG-3′ | 55.3 °C |
| HIF-1A rev | 5′-TGGGTAGGAGATGGAGATGC-3′ | 59.3 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigro, E.; Mallardo, M.; Polito, R.; Scialò, F.; Bianco, A.; Daniele, A. Adiponectin and Leptin Exert Antagonizing Effects on HUVEC Tube Formation and Migration Modulating the Expression of CXCL1, VEGF, MMP-2 and MMP-9. Int. J. Mol. Sci. 2021, 22, 7516. https://doi.org/10.3390/ijms22147516
Nigro E, Mallardo M, Polito R, Scialò F, Bianco A, Daniele A. Adiponectin and Leptin Exert Antagonizing Effects on HUVEC Tube Formation and Migration Modulating the Expression of CXCL1, VEGF, MMP-2 and MMP-9. International Journal of Molecular Sciences. 2021; 22(14):7516. https://doi.org/10.3390/ijms22147516
Chicago/Turabian StyleNigro, Ersilia, Marta Mallardo, Rita Polito, Filippo Scialò, Andrea Bianco, and Aurora Daniele. 2021. "Adiponectin and Leptin Exert Antagonizing Effects on HUVEC Tube Formation and Migration Modulating the Expression of CXCL1, VEGF, MMP-2 and MMP-9" International Journal of Molecular Sciences 22, no. 14: 7516. https://doi.org/10.3390/ijms22147516