Peripheral Opioid Receptor Blockade Enhances Epithelial Damage in Piroxicam-Accelerated Colitis in IL-10-Deficient Mice
Abstract
:1. Introduction
2. Results
2.1. Il-10-/- Mice Treated with Piroxicam Develop Colitis
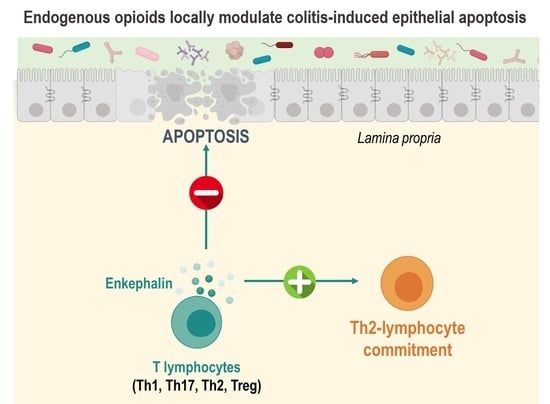
2.2. Peripheral Opioid Receptor Blockade by Naloxone-Methiodide Increases Intestinal Epithelial Cell Apoptosis in Il-10-/- Mice with Colitis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Mice and Naloxone-Methiodide (NLX-Meth) Administration
5.2. Macroscopic Assessment of Colonic Injury
5.3. Histological Assessment of Colonic Injury
5.4. Intestinal Epithelial Cell Apoptosis Assessment
5.5. Real-Time Quantitative PCR Analysis
5.6. Assessment of Intestinal Paracellular Permeability In Vivo
5.7. Isolation of Lamina Propria Mononuclear Cells
5.8. Cytofluorometric Analysis
5.9. Quantification of Polyunsaturated Fatty Acids (PUFA) and Their Metabolites
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basso, L.; Boue, J.; Auge, C.; Deraison, C.; Blanpied, C.; Cenac, N.; Lluel, P.; Vergnolle, N.; Dietrich, G. Mobilization of CD4+ T lymphocytes in inflamed mucosa reduces pain in colitis mice: Toward a vaccinal strategy to alleviate inflammatory visceral pain. Pain 2018, 159, 331–341. [Google Scholar] [CrossRef]
- Boue, J.; Basso, L.; Cenac, N.; Blanpied, C.; Rolli-Derkinderen, M.; Neunlist, M.; Vergnolle, N.; Dietrich, G. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4+ T cells in mice. Gastroenterology 2014, 146, 166–175. [Google Scholar] [CrossRef]
- Hughes, P.A.; Harrington, A.M.; Castro, J.; Liebregts, T.; Adam, B.; Grasby, D.J.; Isaacs, N.J.; Maldeniya, L.; Martin, C.M.; Persson, J.; et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 2013, 62, 1456–1465. [Google Scholar] [CrossRef]
- Valdez-Morales, E.; Guerrero-Alba, R.; Ochoa-Cortes, F.; Benson, J.; Spreadbury, I.; Hurlbut, D.; Miranda-Morales, M.; Lomax, A.E.; Vanner, S. Release of endogenous opioids during a chronic IBD model suppresses the excitability of colonic DRG neurons. Neurogastroenterol. Motil. 2013, 25, 39–46.e34. [Google Scholar] [CrossRef] [PubMed]
- Verma-Gandhu, M.; Bercik, P.; Motomura, Y.; Verdu, E.F.; Khan, W.I.; Blennerhassett, P.A.; Wang, L.; El-Sharkawy, R.T.; Collins, S.M. CD4+ T-cell modulation of visceral nociception in mice. Gastroenterology 2006, 130, 1721–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma-Gandhu, M.; Verdu, E.F.; Bercik, P.; Blennerhassett, P.A.; Al-Mutawaly, N.; Ghia, J.E.; Collins, S.M. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut 2007, 56, 358–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boue, J.; Blanpied, C.; Brousset, P.; Vergnolle, N.; Dietrich, G. Endogenous opioid-mediated analgesia is dependent on adaptive T cell response in mice. J. Immunol. 2011, 186, 5078–5084. [Google Scholar] [CrossRef]
- Boue, J.; Blanpied, C.; Djata-Cabral, M.; Pelletier, L.; Vergnolle, N.; Dietrich, G. Immune conditions associated with CD4+ T effector-induced opioid release and analgesia. Pain 2012, 153, 485–493. [Google Scholar] [CrossRef]
- Brack, A.; Rittner, H.L.; Machelska, H.; Shaqura, M.; Mousa, S.A.; Labuz, D.; Zollner, C.; Schafer, M.; Stein, C. Endogenous peripheral antinociception in early inflammation is not limited by the number of opioid-containing leukocytes but by opioid receptor expression. Pain 2004, 108, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Alba, R.; Valdez-Morales, E.E.; Jimenez-Vargas, N.N.; Bron, R.; Poole, D.; Reed, D.; Castro, J.; Campaniello, M.; Hughes, P.A.; Brierley, S.M.; et al. Co-expression of mu and delta opioid receptors by mouse colonic nociceptors. Br. J. Pharmacol. 2018, 175, 2622–2634. [Google Scholar] [CrossRef] [Green Version]
- Machelska, H.; Celik, M.O. Immune cell-mediated opioid analgesia. Immunol. Lett. 2020, 227, 48–59. [Google Scholar] [CrossRef]
- Stein, C.; Schafer, M.; Machelska, H. Attacking pain at its source: New perspectives on opioids. Nat. Med. 2003, 9, 1003–1008. [Google Scholar] [CrossRef]
- Basso, L.; Benamar, M.; Mas-Orea, X.; Deraison, C.; Blanpied, C.; Cenac, N.; Saoudi, A.; Dietrich, G. Endogenous control of inflammatory visceral pain by T cell-derived opioids in IL-10-deficient mice. Neurogastroenterol. Motil. 2020, 32, e13743. [Google Scholar] [CrossRef]
- Basso, L.; Bourreille, A.; Dietrich, G. Intestinal inflammation and pain management. Curr. Opin. Pharmacol. 2015, 25, 50–55. [Google Scholar] [CrossRef]
- Carbone, S.E.; Poole, D.P. Inflammation without pain: Immune-derived opioids hold the key. Neurogastroenterol. Motil. 2020, 32, e13787. [Google Scholar] [CrossRef] [PubMed]
- Stein, C. New concepts in opioid analgesia. Expert Opin. Investig. Drugs 2018, 27, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lepetre-Mouelhi, S.; Gautier, A.; Mura, S.; Cailleau, C.; Coudore, F.; Hamon, M.; Couvreur, P. A new painkiller nanomedicine to bypass the blood-brain barrier and the use of morphine. Sci. Adv. 2019, 5, eaau5148. [Google Scholar] [CrossRef] [Green Version]
- Mace, G.; Blanpied, C.; Emorine, L.J.; Druet, P.; Dietrich, G. Morphine-like activity of natural human IgG autoantibodies is because of binding to the first and third extracellular loops of the mu-opioid receptor. J. Biol. Chem. 1999, 274, 20079–20082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binning, A.R.; Przesmycki, K.; Sowinski, P.; Morrison, L.M.; Smith, T.W.; Marcus, P.; Lees, J.P.; Dahan, A. A randomised controlled trial on the efficacy and side-effect profile (nausea/vomiting/sedation) of morphine-6-glucuronide versus morphine for post-operative pain relief after major abdominal surgery. Eur. J. Pain 2011, 15, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Cabot, P.J. Targeted nanoparticles that mimic immune cells in pain control inducing analgesic and anti-inflammatory actions: A potential novel treatment of acute and chronic pain condition. Pain Physician 2013, 16, E199–E216. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gaztelumendi, A.; Spahn, V.; Labuz, D.; Machelska, H.; Stein, C. Analgesic effects of a novel pH-dependent mu-opioid receptor agonist in models of neuropathic and abdominal pain. Pain 2018, 159, 2277–2284. [Google Scholar] [CrossRef]
- Spahn, V.; Del Vecchio, G.; Labuz, D.; Rodriguez-Gaztelumendi, A.; Massaly, N.; Temp, J.; Durmaz, V.; Sabri, P.; Reidelbach, M.; Machelska, H.; et al. A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 2017, 355, 966–969. [Google Scholar] [CrossRef]
- Jimenez-Vargas, N.N.; Yu, Y.; Jensen, D.D.; Bok, D.D.; Wisdom, M.; Latorre, R.; Lopez, C.; Jaramillo-Polanco, J.O.; Degro, C.; Guzman-Rodriguez, M.; et al. Agonist that activates the micro-opioid receptor in acidified microenvironments inhibits colitis pain without side effects. Gut 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Auge, C.; Basso, L.; Blanpied, C.; Vergnolle, N.; Game, X.; Chabot, S.; Lluel, P.; Dietrich, G. Pain management in a model of Interstitial Cystitis/Bladder Pain Syndrome by a vaccinal strategy. Front. Pain Res. 2021, 2, 642706. [Google Scholar] [CrossRef]
- Burford, N.T.; Traynor, J.R.; Alt, A. Positive allosteric modulators of the mu-opioid receptor: A novel approach for future pain medications. Br. J. Pharmacol. 2015, 172, 277–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reaux-Le Goazigo, A.; Poras, H.; Ben-Dhaou, C.; Ouimet, T.; Baudouin, C.; Wurm, M.; Melik Parsadaniantz, S. Dual enkephalinase inhibitor PL265: A novel topical treatment to alleviate corneal pain and inflammation. Pain 2019, 160, 307–321. [Google Scholar] [CrossRef]
- Anselmi, L.; Huynh, J.; Duraffourd, C.; Jaramillo, I.; Vegezzi, G.; Saccani, F.; Boschetti, E.; Brecha, N.C.; De Giorgio, R.; Sternini, C. Activation of mu opioid receptors modulates inflammation in acute experimental colitis. Neurogastroenterol. Motil. 2015, 27, 509–523. [Google Scholar] [CrossRef] [Green Version]
- Philippe, D.; Dubuquoy, L.; Groux, H.; Brun, V.; Chuoi-Mariot, M.T.; Gaveriaux-Ruff, C.; Colombel, J.F.; Kieffer, B.L.; Desreumaux, P. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J. Clin. Investig. 2003, 111, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- Plein, L.M.; Rittner, H.L. Opioids and the immune system—Friend or foe. Br. J. Pharmacol. 2018, 175, 2717–2725. [Google Scholar] [CrossRef]
- Stein, C.; Kuchler, S. Targeting inflammation and wound healing by opioids. Trends Pharmacol. Sci. 2013, 34, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.J.; Zhang, J.; Weinstock, J.V.; Ismail, H.F.; Earle, K.A.; Alila, H.; Pamukcu, R.; Moore, S.; Lynch, R.G. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology 2002, 123, 1527–1542. [Google Scholar] [CrossRef]
- Hale, L.P.; Gottfried, M.R.; Swidsinski, A. Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflamm. Bowel Dis. 2005, 11, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Holgersen, K.; Kvist, P.H.; Markholst, H.; Hansen, A.K.; Holm, T.L. Characterisation of enterocolitis in the piroxicam-accelerated interleukin-10 knock out mouse—A model mimicking inflammatory bowel disease. J Crohn’s Colitis 2014, 8, 147–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, B.; Escher, J.; Elawad, M.; Lima, R.; Buderus, S.; Kierkus, J.; Guariso, G.; Canioni, D.; Lambot, K.; Talbotec, C.; et al. Phenotypic characterization of very early-onset IBD due to mutations in the IL10, IL10 receptor alpha or beta gene: A survey of the Genius Working Group. Inflamm. Bowel Dis. 2013, 19, 2820–2828. [Google Scholar] [CrossRef]
- Begue, B.; Verdier, J.; Rieux-Laucat, F.; Goulet, O.; Morali, A.; Canioni, D.; Hugot, J.P.; Daussy, C.; Verkarre, V.; Pigneur, B.; et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am. J. Gastroenterol. 2011, 106, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Kotlarz, D.; Beier, R.; Murugan, D.; Diestelhorst, J.; Jensen, O.; Boztug, K.; Pfeifer, D.; Kreipe, H.; Pfister, E.D.; Baumann, U.; et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: Implications for diagnosis and therapy. Gastroenterology 2012, 143, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Madsen, K.L.; Malfair, D.; Gray, D.; Doyle, J.S.; Jewell, L.D.; Fedorak, R.N. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm. Bowel Dis. 1999, 5, 262–270. [Google Scholar] [CrossRef]
- Holgersen, K.; Kvist, P.H.; Hansen, A.K.; Holm, T.L. Predictive validity and immune cell involvement in the pathogenesis of piroxicam-accelerated colitis in interleukin-10 knockout mice. Int. Immunopharmacol. 2014, 21, 137–147. [Google Scholar] [CrossRef]
- Rosen, M.J.; Karns, R.; Vallance, J.E.; Bezold, R.; Waddell, A.; Collins, M.H.; Haberman, Y.; Minar, P.; Baldassano, R.N.; Hyams, J.S.; et al. Mucosal Expression of Type 2 and Type 17 Immune Response Genes Distinguishes Ulcerative Colitis From Colon-Only Crohn’s Disease in Treatment-Naive Pediatric Patients. Gastroenterology 2017, 152, 1345–1357.e1347. [Google Scholar] [CrossRef] [Green Version]
- Saruta, M.; Yu, Q.T.; Fleshner, P.R.; Mantel, P.Y.; Schmidt-Weber, C.B.; Banham, A.H.; Papadakis, K.A. Characterization of FOXP3+CD4+ regulatory T cells in Crohn’s disease. Clin. Immunol. 2007, 125, 281–290. [Google Scholar] [CrossRef]
- Rubtsov, Y.P.; Rasmussen, J.P.; Chi, E.Y.; Fontenot, J.; Castelli, L.; Ye, X.; Treuting, P.; Siewe, L.; Roers, A.; Henderson, W.R., Jr.; et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008, 28, 546–558. [Google Scholar] [CrossRef]
- Sznurkowska, K.L.J.; Bryl, E.; Witkowski, J.M.; Hermann-Okoniewska, B.; Landowski, P.; Kosek, M.; Szlagatys-Sidorkiewicz, A. Enhancement of Circulating and Intestinal T Regulatory Cells and Their Expression of Helios and Neuropilin-1 in Children with Inflammatory Bowel Disease. J. Inflamm. Res. 2020, 13, 995–1005. [Google Scholar] [CrossRef]
- Asseman, C.; Mauze, S.; Leach, M.W.; Coffman, R.L.; Powrie, F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999, 190, 995–1004. [Google Scholar] [CrossRef]
- Basso, L.; Garnier, L.; Bessac, A.; Boue, J.; Blanpied, C.; Cenac, N.; Laffont, S.; Dietrich, G. T-lymphocyte-derived enkephalins reduce Th1/Th17 colitis and associated pain in mice. J. Gastroenterol. 2018, 53, 215–226. [Google Scholar] [CrossRef]
- Roy, S.; Wang, J.; Charboneau, R.; Loh, H.H.; Barke, R.A. Morphine induces CD4+ T cell IL-4 expression through an adenylyl cyclase mechanism independent of the protein kinase A pathway. J. Immunol. 2005, 175, 6361–6367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Wang, J.; Gupta, S.; Charboneau, R.; Loh, H.H.; Barke, R.A. Chronic morphine treatment differentiates T helper cells to Th2 effector cells by modulating transcription factors GATA 3 and T-bet. J. Neuroimmunol. 2004, 147, 78–81. [Google Scholar] [CrossRef]
- Sacerdote, P.; Manfredi, B.; Gaspani, L.; Panerai, A.E. The opioid antagonist naloxone induces a shift from type 2 to type 1 cytokine pattern in BALB/cJ mice. Blood 2000, 95, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Azarang, A.; Mahmoodi, M.; Rajabalian, S.; Shekari, M.A.; Nosratabadi, J.; Rezaei, N. T-helper 1 and 2 serum cytokine assay in chronic opioid addicts. Eur. Cytokine Netw. 2007, 18, 210–214. [Google Scholar] [PubMed]
- Arrieta, M.C.; Madsen, K.; Doyle, J.; Meddings, J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 2009, 58, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreau, F.; Madre, C.; Meinzer, U.; Berrebi, D.; Dussaillant, M.; Merlin, F.; Eckmann, L.; Karin, M.; Sterkers, G.; Bonacorsi, S.; et al. Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer’s patches. Gut 2010, 59, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, P.; Ma, Y.; Chen, H.; Zhou, Y.; Zhang, M.; Chu, Z.; Qin, H. Lactobacillus plantarum prevents the development of colitis in IL-10-deficient mouse by reducing the intestinal permeability. Mol. Biol. Rep. 2011, 38, 1353–1361. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Uronis, J.M.; Jobin, C. Mu opioid signaling protects against acute murine intestinal injury in a manner involving Stat3 signaling. Am. J. Pathol. 2011, 179, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.; Pol, O.; Puig, M.M. Intestinal inflammation enhances the inhibitory effects of opioids on intestinal permeability in mice. J. Pharmacol. Exp. Ther. 2001, 296, 378–387. [Google Scholar] [PubMed]
- Valle, L.; Puig, M.M.; Pol, O. Effects of mu-opioid receptor agonists on intestinal secretion and permeability during acute intestinal inflammation in mice. Eur. J. Pharmacol. 2000, 389, 235–242. [Google Scholar] [CrossRef]
- Harari, Y.; Weisbrodt, N.W.; Moody, F.G. Ileal mucosal response to bacterial toxin challenge. J. Trauma 2000, 49, 306–313. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [Green Version]
- Harari, Y.; Weisbrodt, N.W.; Moody, F.G. The effect of morphine on mast cell-mediated mucosal permeability. Surgery 2006, 139, 54–60. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Ahrens, R.; Osterfeld, H.; Gurish, M.F.; Han, X.; Abrink, M.; Finkelman, F.D.; Pejler, G.; Hogan, S.P. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 22381–22386. [Google Scholar] [CrossRef] [Green Version]
- Franchi, S.; Moretti, S.; Castelli, M.; Lattuada, D.; Scavullo, C.; Panerai, A.E.; Sacerdote, P. Mu opioid receptor activation modulates Toll like receptor 4 in murine macrophages. Brain Behav. Immun. 2012, 26, 480–488. [Google Scholar] [CrossRef]
- Moller, P.; Koretz, K.; Leithauser, F.; Bruderlein, S.; Henne, C.; Quentmeier, A.; Krammer, P.H. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int. J. Cancer 1994, 57, 371–377. [Google Scholar] [CrossRef]
- Park, S.M.; Chen, L.; Zhang, M.; Ashton-Rickardt, P.; Turner, J.R.; Peter, M.E. CD95 is cytoprotective for intestinal epithelial cells in colitis. Inflamm. Bowel Dis. 2010, 16, 1063–1070. [Google Scholar] [CrossRef]
- Jaume, M.; Jacquet, S.; Cavailles, P.; Mace, G.; Stephan, L.; Blanpied, C.; Demur, C.; Brousset, P.; Dietrich, G. Opioid receptor blockade reduces Fas-induced hepatitis in mice. Hepatology 2004, 40, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Charboneau, R.; Barke, R.A.; Loh, H.H.; Roy, S. Mu-opioid receptor mediates chronic restraint stress-induced lymphocyte apoptosis. J. Immunol. 2002, 169, 3630–3636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, D.; Tuthill, D.; Mufson, R.A.; Shi, Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J. Exp. Med. 2000, 191, 1423–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaume, M.; Laffont, S.; Chapey, E.; Blanpied, C.; Dietrich, G. Opioid receptor blockade increases the number of lymphocytes without altering T cell response in draining lymph nodes in vivo. J. Neuroimmunol. 2007, 188, 95–102. [Google Scholar] [CrossRef]
- Benard, A.; Cavailles, P.; Boue, J.; Chapey, E.; Bayry, J.; Blanpied, C.; Meyer, N.; Lamant, L.; Kaveri, S.V.; Brousset, P.; et al. μ-Opioid receptor is induced by IL-13 within lymph nodes from patients with Sezary syndrome. J. Investig. Dermatol. 2010, 130, 1337–1344. [Google Scholar] [CrossRef]
- Jung, C.; Meinzer, U.; Montcuquet, N.; Thachil, E.; Chateau, D.; Thiebaut, R.; Roy, M.; Alnabhani, Z.; Berrebi, D.; Dussaillant, M.; et al. Yersinia pseudotuberculosis disrupts intestinal barrier integrity through hematopoietic TLR-2 signaling. J. Clin. Investig. 2012, 122, 2239–2251. [Google Scholar] [CrossRef]
- Le Faouder, P.; Baillif, V.; Spreadbury, I.; Motta, J.P.; Rousset, P.; Chene, G.; Guigne, C.; Terce, F.; Vanner, S.; Vergnolle, N.; et al. LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J. Chromatogr. B 2013, 932, 123–133. [Google Scholar] [CrossRef]
- Pujo, J.; Petitfils, C.; Le Faouder, P.; Eeckhaut, V.; Payros, G.; Maurel, S.; Perez-Berezo, T.; Van Hul, M.; Barreau, F.; Blanpied, C.; et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 2020, 70, 1088–1097. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mas-Orea, X.; Sebert, M.; Benamar, M.; Petitfils, C.; Blanpied, C.; Saoudi, A.; Deraison, C.; Barreau, F.; Cenac, N.; Dietrich, G. Peripheral Opioid Receptor Blockade Enhances Epithelial Damage in Piroxicam-Accelerated Colitis in IL-10-Deficient Mice. Int. J. Mol. Sci. 2021, 22, 7387. https://doi.org/10.3390/ijms22147387
Mas-Orea X, Sebert M, Benamar M, Petitfils C, Blanpied C, Saoudi A, Deraison C, Barreau F, Cenac N, Dietrich G. Peripheral Opioid Receptor Blockade Enhances Epithelial Damage in Piroxicam-Accelerated Colitis in IL-10-Deficient Mice. International Journal of Molecular Sciences. 2021; 22(14):7387. https://doi.org/10.3390/ijms22147387
Chicago/Turabian StyleMas-Orea, Xavier, Morgane Sebert, Mehdi Benamar, Camille Petitfils, Catherine Blanpied, Abdelhadi Saoudi, Céline Deraison, Frederick Barreau, Nicolas Cenac, and Gilles Dietrich. 2021. "Peripheral Opioid Receptor Blockade Enhances Epithelial Damage in Piroxicam-Accelerated Colitis in IL-10-Deficient Mice" International Journal of Molecular Sciences 22, no. 14: 7387. https://doi.org/10.3390/ijms22147387