Electromagnetic Field (EMF) Radiation Alters Estrogen Release from the Pig Myometrium during the Peri-Implantation Period
Abstract
:1. Introduction
2. Results
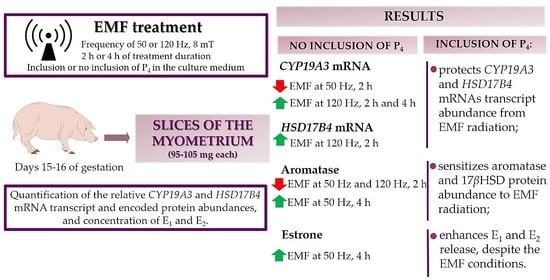
2.1. EMF at 50 and 120 Hz Alter CYP19A3 and HSD17B4 mRNA Transcript Abundance in Myometrial Slices after 2 h and 4 h of In Vitro Incubation in the Presence or Absence of P4
2.2. The Immunolocalization and the Abundance of Aromatase and 17βHSD in Myometrial Slices Treated with EMF at 50 and 120 Hz for 2 h and 4 h with or without the Inclusion of P4
2.3. Effect of EMF at 50 and 120 Hz on E1 and E2 Secretion In Vitro from Myometrial Slices Incubated with or without the Inclusion of P4
2.4. Estrogen Concentration in Uterine Flushings and Blood Plasma
3. Discussion
4. Materials and Methods
4.1. Animals and Collection of Myometrial Tissue
4.2. In Vitro Incubation and EMF Treatment System
4.3. Determination of CYP19A3 and HSD17B4 mRNA Transcript Abundance
4.4. Immunodetection of Aromatase and 17βHSD
4.5. Determination of Aromatase and 17βHSD Protein Abundance
4.6. Determination of E1 and E2 Concentrations in Blood Plasma, Uterine Flushings and the Culture Medium
4.7. Statistical Analysis and Data Presentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geisert, R.D.; Renegar, R.H.; Thatcher, W.W.; Roberts, R.M.; Bazer, F.W. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol. Reprod. 1982, 27, 925–939. [Google Scholar] [CrossRef] [Green Version]
- Ziecik, A.; Waclawik, A.; Kaczmarek, M.; Blitek, A.; Jalali, B.M.; Andronowska, A. Mechanisms for the establishment of pregnancy in the Pig. Reprod. Domest. Anim. 2011, 46, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Ziecik, A.J.; Przygrodzka, E.; Jalali, B.M.; Kaczmarek, M.M. Regulation of the porcine corpus luteum during pregnancy. Reproduction 2018, 156, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Johnson, G.A. Pig blastocyst-uterine interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Waclawik, A.; Kaczmarek, M.M.; Blitek, A.; Kaczynski, P.; Ziecik, A.J. Embryo-maternal dialogue during pregnancy establishment and implantation in the pig. Mol. Reprod. Dev. 2017, 84, 842–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franczak, A. Endometrial and myometrial secretion of androgens and estrone during early pregnancy and luteolysis in pigs. Reprod. Biol. 2008, 8, 213–228. [Google Scholar] [CrossRef]
- Franczak, A.; Kotwica, G. Secretion of estradiol-17β by porcine endometrium and myometrium during early pregnancy and luteolysis. Theriogenology 2008, 69, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Franczak, A.; Kotwica, G. Androgens and estradiol-17β production by porcine uterine cells: In vitro study. Theriogenology 2010, 73, 232–241. [Google Scholar] [CrossRef]
- Franczak, A.; Wojciechowicz, B.; Katwica, G. Novel aspects of cytokine action in porcine uterus—Endometrial and myometrial production of estrone (E1) in the presence of interleukin 1β (IL1β), interleukin 6 (IL6) and tumor necrosis factor (TNFα)—In vitro study. Folia Biol. 2013, 61, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franczak, A.; Wojciechowicz, B.; Kolakowska, J.; Kotwica, G. The effect of interleukin-1β, interleukin-6, and tumor necrosis factor-α on estradiol-17β release in the myometrium: The in vitro study on the pig model. Theriogenology 2014, 81, 266–274. [Google Scholar] [CrossRef]
- Smolinska, N.; Dobrzyn, K.; Kiezun, M.; Szeszko, K.; Maleszka, A.; Kaminski, T. Effect of adiponectin on the steroidogenic acute regulatory protein, P450 side chain cleavage enzyme and 3β-hydroxysteroid dehydrogenase gene expression, progesterone and androstenedione production by the porcine uterus during early pregnancy. J. Physiol. Pharmacol. 2016, 67, 443–456. [Google Scholar] [PubMed]
- Kiezun, M.; Smolinska, N.; Dobrzyn, K.; Szeszko, K.; Rytelewska, E.; Kaminski, T. The effect of orexin A on CYP17A1 and CYP19A3 expression and on oestradiol, oestrone and testosterone secretion in the porcine uterus during early pregnancy and the oestrous cycle. Theriogenology 2017, 90, 129–140. [Google Scholar] [CrossRef]
- Grzesiak, M.; Waszkiewicz, E.; Wojtas, M.; Kowalik, K.; Franczak, A. Expression of vitamin D receptor in the porcine uterus and effect of 1,25(OH)2D3 on progesterone and estradiol-17β secretion by uterine tissues in vitro. Theriogenology 2019, 125, 102–108. [Google Scholar] [CrossRef]
- Kisielewska, K.; Rytelewska, E.; Gudelska, M.; Kiezun, M.; Dobrzyn, K.; Szeszko, K.; Bors, K.; Wyrebek, J.; Kaminski, T.; Smolinska, N. The effect of orexin B on steroidogenic acute regulatory protein, P450 side-chain cleavage enzyme, and 3β-hydroxysteroid dehydrogenase gene expression, and progesterone and androstenedione secretion by the porcine uterus during early pregnancy and the est. J. Anim. Sci. 2019, 97, 851–864. [Google Scholar] [CrossRef]
- Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Kiezun, M.; Dobrzyn, K.; Bors, K.; Wyrebek, J.; Kaminska, B.; Kaminski, T.; Smolinska, N. The effect of orexin a on the StAR, CYP11A1 and HSD3B1 gene expression, as well as progesterone and androstenedione secretion in the porcine uterus during early pregnancy and the oestrous cycle. Theriogenology 2020, 143, 179–190. [Google Scholar] [CrossRef]
- Waszkiewicz, E.M.; Kozlowska, W.; Zmijewska, A.; Franczak, A. Expression of insulin-like growth factor 1 (IGF-1) and epidermal growth factor (EGF) receptors and the effect of IGF-1 and EGF on androgen and estrogen release in the myometrium of pigs—In vitro study. Animals 2020, 10, 915. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz, E.M.; Zmijewska, A.; Kozlowska, W.; Franczak, A. Effects of LH and FSH on androgen and oestrogen release in the myometrium of pigs during the oestrous cycle and early pregnancy. Reprod. Fertil. Dev. 2020, 32, 1200–1211. [Google Scholar] [CrossRef]
- Koziorowska, A.; Waszkiewicz, E.M.; Romerowicz-Misielak, M.; Zglejc-Waszak, K.; Franczak, A. Extremely low-frequency electromagnetic field (EMF) generates alterations in the synthesis and secretion of oestradiol-17β (E2) in uterine tissues: An in vitro study. Theriogenology 2018, 110, 86–95. [Google Scholar] [CrossRef]
- Franczak, A.; Waszkiewicz, E.; Kozlowska, W.; Zmijewska, A.; Koziorowska, A. Consequences of electromagnetic field (EMF) radiation during early pregnancy—Androgen synthesis and release from the myometrium of pigs in vitro. Anim. Reprod. Sci. 2020, 218, 106465. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, W.; Drzewiecka, E.; Zmijewska, A.; Koziorowska, A.; Franczak, A. Effects of electromagnetic field (EMF) radiation on androgen synthesis and release from the pig endometrium during the fetal peri-implantation period. Anim. Reprod. Sci. 2021, 226, 106694. [Google Scholar] [CrossRef] [PubMed]
- Gajšek, P.; Ravazzani, P.; Grellier, J.; Samaras, T.; Bakos, J.; Thuróczy, G. Review of studies concerning electromagnetic field (EMF) exposure assessment in Europe: Low frequency fields (50 Hz–100 kHz). Int. J. Environ. Res. Public Health 2016, 13, 875. [Google Scholar] [CrossRef] [Green Version]
- Koziorowska, A.; Depciuch, J.; Kozioł, K.; Nowak, S.; Lach, K. In vitro study of effects of ELF-EMF on testicular tissues of roe deer (Capreolus capreolus)—FTIR and FT-Raman spectroscopic investigation. Anim. Reprod. Sci. 2020, 213, 106258. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Liu, Y. Effects of exposure to extremely low frequency electromagnetic fields on reproduction of female mice and development of offsprings. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2006, 24, 468–470. [Google Scholar] [PubMed]
- Mansuori, E.; Alihemmati, A.; Mesbahi, A. An overview on the effects of power frequency electromagnetic field exposure on the female reproduction system, pregnancy outcome and fetal development. J. Med. Chem. Sci. 2020, 3, 60–70. [Google Scholar]
- Darwish, S.M.; Darwish, A.; Darwish, D.S. An extremely low-frequency magnetic field can affect CREB protein conformation which may have a role in neuronal activities including memory. J. Phys. Commun. 2020, 4, 1–10. [Google Scholar] [CrossRef]
- Mahaki, H.; Jabarivasal, N.; Sardanian, K.; Zamani, A. Effects of Various Densities of 50 Hz Electromagnetic Field on Serum IL-9, IL-10, and TNF-α Levels. Int. J. Occup. Environ. Med. 2020, 11, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.; Williams, D.H.; Lynch, P.B.; Hanrahan, T.J.; McGeady, T.A.; Austin, F.H.; Boland, M.P.; Roche, J.F. The extent and timing of prenatal loss in gilts. Theriogenology 1991, 36, 655–665. [Google Scholar] [CrossRef]
- Christenson, L.K.; Anderson, L.H.; Ford, S.P.; Farley, D.B. Luteal maintenance during early pregnancy in the pig: Role for prostaglandin E2. Prostaglandins 1994, 47, 61–75. [Google Scholar] [CrossRef]
- Waclawik, A.; Jabbour, H.N.; Blitek, A.; Ziecik, A.J. Estradiol-17β, prostaglandin E2 (PGE2), and the PGE2 receptor are involved in PGE2 positive feedback loop in the porcine endometrium. Endocrinology 2009, 150, 3823–3832. [Google Scholar] [CrossRef] [Green Version]
- Ziecik, A.J. Old, new and the newest concepts of inhibition of luteolysis during early pregnancy in pig. Domest. Anim. Endocrinol. 2002, 23, 265–275. [Google Scholar] [CrossRef]
- Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Bazer, F.W. Progesterone and placental hormone actions on the uterus: Insights from domestic animals. Biol. Reprod. 2004, 71, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Hoorn, M.-L.; Lashley, L. Effectiveness of progesterone in pregnancy complications. Ned. Tijdschr. Geneeskd. 2019, 163, D4395. [Google Scholar] [PubMed]
- Standeven, L.; McEvoy, K.; Osborne, L. Progesterone, Reproduction, and Psychiatric Illness. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 108–126. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L. 28.2 Eukaryotic Transcription and Translation Are Separated in Space and Time. In Biochemistry; W H Freeman: New York, NY, USA, 2002. [Google Scholar]
- Hall, D.P.F. Cytochromes P-450 and the regulation of steroid synthesis. Steroids 1986, 48, 131–196. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [Green Version]
- Makieva, S.; Saunders, P.T.K.; Norman, J.E. Androgens in pregnancy: Roles in parturition. Hum. Reprod. Update 2014, 20, 542–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Hinshelwood, M.M.; Graham-LORENCE, S.; Amarneh, B.; Ito, Y.; Fisher, C.R.; Michael, M.D.; et al. Aromatase cytochrome p450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994, 15, 342–355. [Google Scholar]
- Franczak, A.; Zmijewska, A.; Kurowicka, B.; Wojciechowicz, B.; Kotwica, G. Interleukin 1β-induced synthesis and secretion of prostaglandin E2 in the porcine uterus during various periods of pregnancy and the estrous cycle. J. Physiol. Pharmacol. 2010, 61, 733–742. [Google Scholar]
- Tang, C.; Pan, Y.; Luo, H.; Xiong, W.; Zhu, H.; Ruan, H.; Wang, J.; Zou, C.; Tang, L.; Iguchi, T.; et al. Hedgehog signaling stimulates the conversion of cholesterol to steroids. Cell. Signal. 2015, 27, 487–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, D.B.; Janowski, B.A.; Chen, C.C.; Mendelson, C.R. Progesterone receptor inhibits aromatase and inflammatory response pathways in breast cancer cells via ligand-dependent and ligand-independent mechanisms. Mol. Endocrinol. 2008, 22, 1812–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gry, M.; Rimini, R.; Strömberg, S.; Asplund, A.; Pontén, F.; Uhlén, M.; Nilsson, P. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics 2009, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Bazer, F.W.; Thatcher, W.W. Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2α by the uterine endometrium. Prostaglandins 1977, 14, 397–401. [Google Scholar] [CrossRef]
- Ryan, K.J. Biochemistry of aromatase: Significance to female reproductive physiology. Cancer Res. 1982, 42, 3342–3344. [Google Scholar]
- Mlynarcikova, A.; Fickova, M.; Scsukova, S. Impact of endocrine disruptors on ovarian steroidogenesis. Endocr. Regul. 2014, 48, 201–224. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Verma, P.; Singh, K. Immune-endocrine crosstalk during pregnancy. Gen. Comp. Endocrinol. 2017, 242, 18–23. [Google Scholar] [CrossRef]
- Conley, A.J.; Ford, S.P. Direct luteotrophic effect of oestradiol-17β on pig corpora lutea. J. Reprod. Fertil. 1989, 87, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisert, R.D.; Ross, J.W.; Ashworth, M.D.; White, F.J.; Johnson, G.A.; DeSilva, U. Maternal recognition of pregnancy signal or endocrine disruptor: The two faces of oestrogen during establishment of pregnancy in the pig. Soc. Reprod. Fertil. Suppl. 2006, 62, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowicz, B.; Kotwica, G.; Zglejc, K.; Waszkiewicz, E.; Franczak, A. Expression of 17β-hydroxysteroid dehydrogenase and the effects of LH, FSH and prolactin on oestrone and 17β-oestradiol secretion in the endometrium of pigs during early pregnancy and the oestrous cycle. Reprod. Fertil. Dev. 2017, 29, 975–984. [Google Scholar] [CrossRef]
- De Sousa Abreu, R.; Penalva, L.O.; Marcotte, E.M.; Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009, 5, 1512–1526. [Google Scholar] [CrossRef] [Green Version]
- Akins, E.L.; Morrissette, M.C. Gross ovarian changes during estrous cycle of swine. Am. J. Vet. Res. 1968, 29, 1953–1957. [Google Scholar] [PubMed]
- Ryan, D.P.; Yaakub, H.; Harrington, D.; Lynch, P.B. Follicular development during early pregnancy and the estrous cycle of the sow. Theriogenology 1994, 42, 623–632. [Google Scholar] [CrossRef]
- Dantzer, V. Electron microscopy of the initial stages of placentation in the pig. Anat. Embryol. 1985, 172, 281–293. [Google Scholar] [CrossRef]
- Oestrup, O.; Hall, V.; Petkov, S.G.; Wolf, X.A.; Hyldig, S.; Hyttel, P. From zygote to implantation: Morphological and molecular dynamics during embryo development in the pig. Reprod. Domest. Anim. 2009, 44, 39–49. [Google Scholar] [CrossRef]
- Koziorowska, A.; Pasiud, E.; Fila, M.; Romerowicz-Misielak, M. The impact of electromagnetic field at a frequency of 50 Hz and a magnetic induction of 2.5 mT on viability of pineal cells in vitro. J. Biol. Regul. Homeost. Agents 2016, 30, 1067–1072. [Google Scholar] [PubMed]
- Grandolfo, M. Protection of workers from power frequency electric and magnetic fields: A practical guide. In Occupational Safety and Health Series; International Labour Office: Geneva, Switzerland, 1994; p. 69. ISBN 9789221082613. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ciereszko, R.; Renata, C. Radioimmunoassay of Steroid Hormones in Biological Fluids. In Demonstrations and Methods; Przala, J., Ed.; University of Warmia and Mazury Press: Olsztyn, Poland, 2009. [Google Scholar]
- Szafrańska, B.; Ziecik, A.; Okrasa, S. Primary antisera against selected steroids or proteins and secondary antisera against gamma-globulins—An available tool for studies of reproductive processes. Reprod. Biol. 2002, 2, 187–204. [Google Scholar]






| CYP19A3 mRNA | HSD17B4 mRNA | |||
|---|---|---|---|---|
| Factor | F | p | F | p |
| Treatment with an EMF 1 | 0.64626 | 0.530705 | 0.2970 | 0.744938 |
| Duration of EMF treatment 2 | 1.78356 | 0.191135 | 2.5835 | 0.117231 |
| Inclusion of P4 in medium 3 | 0.15396 | 0.697379 | 1.9135 | 0.175597 |
| Treatment with an EMF × Duration of EMF treatment | 4.01165 | 0.027886 | 11.1303 | 0.000191 |
| Treatment with an EMF × The inclusion of P4 in medium | 0.89975 | 0.416711 | 3.0523 | 0.060373 |
| Duration of EMF treatment × The inclusion of P4 in medium | 16.93924 | 0.000253 | 0.7142 | 0.403948 |
| Treatment with an EMF × Duration of EMF treatment × The inclusion of P4 in medium | 7.47019 | 0.002176 | 3.1239 | 0.056823 |
| Aromatase | 17βHSD | |||
|---|---|---|---|---|
| Factor | F | p | F | p |
| Treatment with an EMF 1 | 0.3757 | 0.689390 | 6.2235 | 0.004293 |
| Duration of EMF treatment 2 | 7.7595 | 0.008376 | 0.4887 | 0.488369 |
| Inclusion of P4 in medium 3 | 9.1335 | 0.004537 | 0.0361 | 0.850231 |
| Treatment with an EMF × Duration of EMF treatment | 12.5524 | 0.000069 | 2.7590 | 0.074853 |
| Treatment with an EMF × The inclusion of P4 in medium | 4.8927 | 0.013022 | 7.7074 | 0.001408 |
| Duration of EMF treatment × The inclusion of P4 in medium | 1.6380 | 0.208562 | 1.5019 | 0.227210 |
| Treatment with an EMF × Duration of EMF treatment × The inclusion of P4 in medium | 5.7070 | 0.006915 | 5.6520 | 0.006703 |
| Estrone | Estradiol-17β | |||
|---|---|---|---|---|
| Factor | F | p | F | p |
| Treatment with an EMF 1 | 12.20 | 0.000012 | 2.09 | 0.127233 |
| Duration of EMF treatment 2 | 0.78 | 0.378135 | 2.30 | 0.131376 |
| Inclusion of P4 in medium 3 | 453.97 | 0.000000 | 773.30 | 0.000000 |
| Treatment with an EMF × Duration of EMF treatment | 6.75 | 0.001536 | 0.05 | 0.955168 |
| Treatment with an EMF × The inclusion of P4 in medium | 0.64 | 0.526061 | 1.26 | 0.287898 |
| Duration of EMF treatment × The inclusion of P4 in medium | 0.10 | 0.755648 | 5.63 | 0.019079 |
| Treatment with an EMF × Duration of EMF treatment × The inclusion of P4 in medium | 1.44 | 0.239492 | 0.22 | 0.806063 |
| Target Gene Symbol | Target Gene Name | Taq Man Assay IDs |
|---|---|---|
| CYP19A3 | cytochrome P450arom | Ss03384905_uH |
| HSD17B4 | 17β-hydroxysteroid dehydrogenase | Ss04245958_g1 |
| Reference genes | ||
| ACTB | β-actin | Ss03376081_u1 |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase | Ss03374854_g1 |
| Name | Type | Catalog No./Company | Host | Concentration |
|---|---|---|---|---|
| Anti-β-actin | Primary | A2066 (Sigma Aldrich) | Rabbit | 2 μg/mL |
| Anti-aromatase | Primary | A7981 (Sigma Aldrich) | Rabbit | 1 μg/mL |
| Anti-17βHSD | Primary | Orb137855 (Biorbyt) | Rabbit | 1 μg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzewiecka, E.M.; Kozlowska, W.; Zmijewska, A.; Wydorski, P.J.; Franczak, A. Electromagnetic Field (EMF) Radiation Alters Estrogen Release from the Pig Myometrium during the Peri-Implantation Period. Int. J. Mol. Sci. 2021, 22, 2920. https://doi.org/10.3390/ijms22062920
Drzewiecka EM, Kozlowska W, Zmijewska A, Wydorski PJ, Franczak A. Electromagnetic Field (EMF) Radiation Alters Estrogen Release from the Pig Myometrium during the Peri-Implantation Period. International Journal of Molecular Sciences. 2021; 22(6):2920. https://doi.org/10.3390/ijms22062920
Chicago/Turabian StyleDrzewiecka, Ewa Monika, Wiktoria Kozlowska, Agata Zmijewska, Pawel Jozef Wydorski, and Anita Franczak. 2021. "Electromagnetic Field (EMF) Radiation Alters Estrogen Release from the Pig Myometrium during the Peri-Implantation Period" International Journal of Molecular Sciences 22, no. 6: 2920. https://doi.org/10.3390/ijms22062920