The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment—A Narrative Review
Abstract
:1. Introduction
2. Methods
3. Isoflavones
3.1. Classification and Metabolism of Isoflavones
3.2. Mechanisms of Isoflavones Action
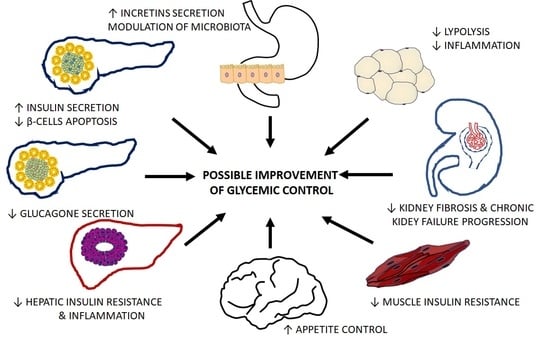
4. Influence of Isoflavones on the Function of Organs Critical for Diabetes Development and Progression
4.1. Pancreatic Islets
4.2. Liver
4.3. Muscle
4.4. Adipose Tissue
4.5. Kidneys
4.6. Gastrointestinal Tract
4.7. Brain
5. Isoflavones in Management of Type 2 Diabetes
5.1. Epidemiological Studies
5.2. Interventional Studies
6. Final Remarks and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 30 November 2020).
- Chu, D.T.; Minh Nguyet, N.T.; Dinh, T.C.; Thai Lien, N.V.; Nguyen, K.H.; Nhu Ngoc, V.T.; Tao, Y.; Son, L.H.; Le, D.H.; Nga, V.B.; et al. An update on physical health and economic consequences of overweight and obesity. Diabetes Metab. Syndr. 2018, 12, 1095–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Frankenfeld, C.L.; Atkinson, C.; Thomas, W.K.; Gonzalez, A.; Jokela, T.; Wähälä, K.; Schwartz, S.M.; Li, S.S.; Lampe, J.W. High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart. Br. J. Nutr. 2009, 94, 873–876. [Google Scholar] [CrossRef]
- Ricketts, M.L.; Moore, D.D.; Banz, W.J.; Mezei, O.; Shay, N.F. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. J. Nutr. Biochem. 2005, 16, 321–330. [Google Scholar] [CrossRef]
- Chadha, R.; Bhalla, Y.; Jain, A.; Chadha, K.; Karan, M. Dietary Soy Isoflavone: A Mechanistic Insight. Nat. Prod. Commun. 2017, 12, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Burns, K.A.; Korach, K.S. Estrogen receptors and human disease: An update. Arch. Toxicol. 2012, 86, 1491–1504. [Google Scholar] [CrossRef] [Green Version]
- Foryst-Ludwig, A.; Kintscher, U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J. Steroid Biochem. Mol. Biol. 2010, 122, 74–81. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Chiarelli, F.; Di Marzio, D. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: Current evidence and future perspectives. Vasc. Health Risk Manag. 2008, 4, 297–304. [Google Scholar] [PubMed] [Green Version]
- Patel, R.P.; Barnes, S. Isoflavones and PPAR Signaling: A Critical Target in Cardiovascular, Metastatic, and Metabolic Disease. PPAR Res. 2010, 2010, 153252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medjakovic, S.; Jungbauer, A. Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J. Steroid Biochem. Mol. Biol. 2008, 108, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Cammarata, P.R.; Baines, C.P.; Yager, J.D. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim. Biophys. Acta 2009, 1793, 1540–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Q.C.; Li, Y.L.; Qin, Y.F.; Quarles, L.D.; Xu, K.K.; Li, R.; Zhou, H.H.; Xiao, Z.S. Inhibition of adipocyte differentiation by phytoestrogen genistein through a potential downregulation of extracellular signal-regulated kinases 1/2 activity. J. Cell. Biochem. 2008, 104, 1853–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosmann, B.; Behl, C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl. Acad. Sci. USA 1999, 96, 8867–8872. [Google Scholar] [CrossRef] [Green Version]
- Park, C.E.; Yun, H.; Lee, E.B.; Min, B.I.; Bae, H.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J. Med. Food 2010, 13, 815–820. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Mao, J.; Li, H.; Wang, M.; Zhang, H.; Li, H.; Chen, W. Genistein Ameliorates Non-alcoholic Fatty Liver Disease by Targeting the Thromboxane A2 Pathway. J. Agric. Food Chem. 2018, 66, 5853–5859. [Google Scholar] [CrossRef]
- Kim, E.K.; Kwon, K.B.; Song, M.Y.; Seo, S.W.; Park, S.J.; Ka, S.O.; Na, L.; Kim, K.A.; Ryu, D.G.; So, H.S.; et al. Genistein protects pancreatic beta cells against cytokine-mediated toxicity. Mol. Cell. Endocrinol. 2007, 278, 18–28. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Pang, X.; Yang, J.; Yu, H.; Zhang, Y.; Zhou, H.; Zhao, J. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol. Lett. 2017, 277, 115–122. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharm. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pudenz, M.; Roth, K.; Gerhauser, C. Impact of soy isoflavones on the epigenome in cancer prevention. Nutrients 2014, 6, 4218–4272. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.D.; Ho, S.M.; Zhang, L.; Chen, J.; Cui, W.; Slager, R.; Gray, S.; Hawkins, G.A.; Medvedovic, M.; Wagner, J.D. Epigenetic changes with dietary soy in cynomolgus monkeys. PLoS ONE 2011, 6, e26791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Zhu, W.; Shi, H.; Hewett, J.E.; Ruhlen, R.L.; MacDonald, R.S.; Rottinghaus, G.E.; Chen, Y.C.; Sauter, E.R. Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr. Cancer 2009, 61, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.; Pang, X.; Yang, J.; Yu, H.; Zhang, Y.; Zhou, H.; Zhao, J. Genistein protects against ox-LDL-induced inflammation through microRNA-155/SOCS1-mediated repression of NF-ĸB signaling pathway in HUVECs. Inflammation 2017, 40, 1450–1459. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Liu, L.; Guo, Z.; Wang, S.; Chen, L.; Zheng, T.; Fan, Y.; Tan, Y.; Jiang, D.; et al. miR-222 is involved in the regulation of genistein on skeletal muscle fiber type. J. Nutr. Biochem. 2020, 80, 108320. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Wang, S.; Guo, Z.; Zheng, T.; Tan, Y.; Fan, Y.; Liu, L.; Chen, L.; Jiang, A.; et al. Genistein inhibits high fat diet-induced obesity through miR-222 by targeting BTG2 and adipor1. Food Funct. 2020, 11, 2418–2426. [Google Scholar] [CrossRef]
- Zhou, Q.; Melton, D.A. Pancreas regeneration. Nature 2018, 557, 351–358. [Google Scholar] [CrossRef]
- Cersosimo, E.; Triplitt, C.; Solis-Herrera, C.; Mandarino, L.J.; DeFronzo, R.A. Pathogenesis of Type 2 Diabetes Mellitus. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Zhong, W.W.; Liu, Y.; Li, C.L. Mechanisms of genistein protection on pancreas cell damage in high glucose condition. Intern. Med. 2011, 50, 2129–2134. [Google Scholar] [CrossRef] [Green Version]
- Sorenson, R.L.; Brelje, T.C.; Roth, C. Effect of tyrosine kinase inhibitors on islets of Langerhans: Evidence for tyrosine kinases in the regulation of insulin secretion. Endocrinology 1994, 134, 1975–1978. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, W.; Zhen, W.; Lum, H.; Nadler, J.; Bassaganya-Riera, J.; Jia, Z.; Wang, Y.; Misra, H.; Liu, D. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 2010, 151, 3026–3037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, J.C.; Plant, T.D.; Gilon, P.; Detimary, P.; Nenquin, M.; Henquin, J.C. Multiple effects and stimulation of insulin secretion by the tyrosine kinase inhibitor genistein in normal mouse islets. Br. J. Pharmacol. 1995, 114, 872–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zhen, W.; Yang, Z.; Carter, J.D.; Si, H.; Reynolds, K.A. Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes 2006, 55, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Liu, D. Long-term exposure to genistein improves insulin secretory function of pancreatic beta-cells. Eur. J. Pharmacol. 2009, 616, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006, 79, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- El-Kordy, E.A.; Alshahrani, A.M. Effect of genistein, a natural soy isoflavone, on pancreatic β-cells of streptozotocin-induced diabetic rats: Histological and immunohistochemical study. J. Microsc. Ultrastruct. 2015, 3, 108–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.; Gilbert, E.R.; Pfeiffer, L.; Zhang, Y.; Fu, Y.; Liu, D. Genistein ameliorates hyperglycemia in a mouse model of nongenetic type 2 diabetes. Appl. Physiol. Nutr. Metab. 2012, 37, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Ae Park, S.; Choi, M.S.; Cho, S.Y.; Seo, J.S.; Jung, U.J.; Kim, M.J.; Sung, M.K.; Park, Y.B.; Lee, M.K. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006, 79, 1207–1213. [Google Scholar] [CrossRef]
- Yousefi, H.; Alihemmati, A.; Karimi, P.; Alipour, M.R.; Habibi, P.; Ahmadiasl, N. Effect of genistein on expression of pancreatic SIRT1, inflammatory cytokines and histological changes in ovariectomized diabetic rat. Iran. J. Basic Med. Sci. 2017, 20, 423–429. [Google Scholar]
- Horiuchi, H.; Harada, N.; Adachi, T.; Nakano, Y.; Inui, H.; Yamaji, R. S-equol enantioselectively activates cAMP-protein kinase A signaling and reduces alloxan-induced cell death in INS-1 pancreatic β-cells. J. Nutr. Sci. Vitaminol. 2014, 60, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Lang, H.; Wang, L.; Liu, K.; Zhou, Y.; Mi, M. S-Equol ameliorates insulin secretion failure through Chrebp/Txnip signaling via modulating PKA/PP2A activities. Nutr. Metab. (Lond) 2020, 17, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, G.; Tian, W.; Huan, M.; Chen, J.; Fu, H. Formononetin exhibits anti-hyperglycemic activity in alloxan-induced type 1 diabetic mice. Exp. Biol. Med. (Maywood) 2017, 242, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin Treatment in Type 2 Diabetic Rats Reduces Insulin Resistance and Hyperglycemia. Front. Pharmacol. 2018, 9, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhu, Y.; Gao, L.; Yin, H.; Xie, Z.; Wang, D.; Zhu, Z.; Han, X. Formononetin attenuates IL-1β-induced apoptosis and NF-κB activation in INS-1 cells. Molecules 2012, 17, 10052–10064. [Google Scholar] [CrossRef] [Green Version]
- Harini, R.; Ezhumalai, M.; Pugalendi, K.V. Antihyperglycemic effect of biochanin A, a soy isoflavone, on streptozotocin-diabetic rats. Eur. J. Pharmacol. 2012, 676, 89–94. [Google Scholar] [CrossRef]
- Oza, M.J.; Kulkarni, Y.A. Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed. Pharmacother. 2018, 107, 1119–1127. [Google Scholar] [CrossRef]
- Haitian, M.; Xijie, L. Effect of daidzein and cystamine on the blood concentration of insulin and glucagon in geese. J. North. Agric. Univ. 2002, 33, 251–255. [Google Scholar]
- Guichun, Z.; Xijie, L. Effect of dietary addition daidzein on insulin glucagon in blood plasma and in liver malic-dehydrogenase in the Broilers. J. North. Agric. Univ. 2003, 34, 52–55. [Google Scholar]
- Rockwood, S.; Mason, D.; Lord, R.; Lamar, P.; Prozialeck, W.; Al-Nakkash, L. Genistein diet improves body weight, serum glucose and triglyceride levels in both male and female ob/ob mice. Diabetes Metab. Syndr. Obes 2019, 12, 2011–2021. [Google Scholar] [CrossRef] [Green Version]
- Lomonaco, R.; Bril, F.; Portillo-Sanchez, P.; Ortiz-Lopez, C.; Orsak, B.; Biernacki, D.; Lo, M.; Suman, A.; Weber, M.H.; Cusi, K. Metabolic Impact of Nonalcoholic Steatohepatitis in Obese Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Igarashi, K.; Yu, C. Anti-obese and anti-diabetic effects of a mixture of daidzin and glycitin on C57BL/6J mice fed with a high-fat diet. Biosci. Biotechnol. Biochem. 2015, 79, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Jia, Q.; Mehmood, S.; Ma, S.; Liu, X. Genistein ameliorates inflammation and insulin resistance through mediation of gut microbiota composition in type 2 diabetic mice. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Salih, S.; Nallasamy, P.; Muniyandi, P.; Periyasami, V.; Carani Venkatraman, A. Genistein improves liver function and attenuates non-alcoholic fatty liver disease in a rat model of insulin resistance. J. Diabetes 2009, 1, 278–287. [Google Scholar] [CrossRef]
- Arjunan, S.; Thangaiyan, R.; Balaraman, D. Biochanin A, a soy isoflavone, diminishes insulin resistance by modulating insulin-signalling pathway in high-fat diet-induced diabetic mice. Arch. Physiol. Biochem. 2020, 1–7. [Google Scholar] [CrossRef]
- Crespillo, A.; Alonso, M.; Vida, M.; Pavón, F.J.; Serrano, A.; Rivera, P.; Romero-Zerbo, Y.; Fernández-Llebrez, P.; Martínez, A.; Pérez-Valero, V.; et al. Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 2011, 164, 1899–1915. [Google Scholar] [CrossRef] [Green Version]
- Bell, A.; Korourian, S.; Zeng, H.; Phelps, J.; Hakkak, R. A diet containing a high- versus low-daidzein level does not protect against liver steatosis in the obese Zucker rat model. Food Funct. 2017, 8, 1293–1298. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, J.S.; Jung, J.W.; Byun, K.W.; Kang, K.S.; Lee, Y.S. Daidzein supplementation prevents non-alcoholic fatty liver disease through alternation of hepatic gene expression profiles and adipocyte metabolism. Int. J. Obes. 2011, 35, 1019–1030. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Liu, H.; Jiang, Z. Genistein ameliorates fat accumulation through AMPK activation in fatty acid-induced BRL cells. J. Food Sci. 2017, 82, 2719–2725. [Google Scholar] [CrossRef]
- Yoo, N.Y.; Jeon, S.; Nam, Y.; Park, Y.J.; Won, S.B.; Kwon, Y.H. Dietary Supplementation of Genistein Alleviates Liver Inflammation and Fibrosis Mediated by a Methionine-Choline-Deficient Diet in db/db Mice. J. Agric. Food Chem. 2015, 63, 4305–4311. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, K.S.; Lee, Y.S. The inhibitory effect of genistein on hepatic steatosis is linked to visceral adipocyte metabolism in mice with diet-induced non-alcoholic fatty liver disease. Br. J. Nutr. 2010, 104, 1333–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.S.; Hur, H.J.; Kim, S.H.; Park, S.J.; Hong, M.J.; Sung, M.J.; Kwon, D.Y.; Kim, M.S. Biochanin A improves hepatic steatosis and insulin resistance by regulating the hepatic lipid and glucose metabolic pathways in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Li, X.; Wang, Q.; Yan, M.; Zhang, H.; Zhao, T.; Zhang, N.; Zhang, P.; Peng, L.; et al. Formononetin alleviates hepatic steatosis by facilitating TFEB-mediated lysosome biogenesis and lipophagy. J. Nutr. Biochem. 2019, 73, 108214. [Google Scholar] [CrossRef] [PubMed]
- Gautam, J.; Khedgikar, V.; Kushwaha, P.; Choudhary, D.; Nagar, G.K.; Dev, K.; Dixit, P.; Singh, D.; Maurya, R.; Trivedi, R. Formononetin, an isoflavone, activates AMP-activated protein kinase/β-catenin signalling to inhibit adipogenesis and rescues C57BL/6 mice from high-fat diet-induced obesity and bone loss. Br. J. Nutr. 2017, 117, 645–661. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.; Schjoldager, J.G.; Tortzen, C.G.; Vegge, A.; Hufeldt, M.R.; Skaanild, M.T.; Vogensen, F.K.; Kristiansen, K.; Hansen, A.K.; Nielsen, J. 2-heptyl-formononetin increases cholesterol and induces hepatic steatosis in mice. Biomed. Res. Int 2013, 2013, 926942. [Google Scholar] [CrossRef] [Green Version]
- Xin, X.; Chen, C.; Hu, Y.Y.; Feng, Q. Protective effect of genistein on nonalcoholic fatty liver disease (NAFLD). Biomed. Pharmacother. 2019, 117, 109047. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Gunnarsson, R.; Björkman, O.; Olsson, M.; Wahren, J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J. Clin. Investig. 1985, 76, 149–155. [Google Scholar] [CrossRef]
- Utriainen, T.; Takala, T.; Luotolahti, M.; Rönnemaa, T.; Laine, H.; Ruotsalainen, U.; Haaparanta, M.; Nuutila, P.; Yki-Järvinen, H. Insulin resistance characterizes glucose uptake in skeletal muscle but not in the heart in NIDDM. Diabetologia 1998, 41, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Palacios-González, B.; Zarain-Herzberg, A.; Flores-Galicia, I.; Noriega, L.G.; Alemán-Escondrillas, G.; Zariñan, T.; Ulloa-Aguirre, A.; Torres, N.; Tovar, A.R. Genistein stimulates fatty acid oxidation in a leptin receptor-independent manner through the JAK2-mediated phosphorylation and activation of AMPK in skeletal muscle. Biochem. Biophys. Acta 2014, 1841, 132–140. [Google Scholar] [CrossRef]
- Van Bree, B.W.; Lenaers, E.; Nabben, M.; Briedé, J.J.; Jörgensen, J.A.; Schaart, G.; Schrauwen, P.; Hoeks, J.; Hesselink, M.K. A genistein-enriched diet neither improves skeletal muscle oxidative capacity nor prevents the transition towards advanced insulin resistance in ZDF rats. Sci. Rep. 2016, 6, 22854. [Google Scholar] [CrossRef]
- Wagner, J.D.; Zhang, L.; Shadoan, M.K.; Kavanagh, K.; Chen, H.; Tresnasari, K.; Kaplan, J.R.; Adams, M.R. Effects of soy protein and isoflavones on insulin resistance and adiponectin in male monkeys. Metabolism 2008, 57, S24–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, M.; Naka, A.; Sakamoto, Y.; Shibasaki, A.; Toh, M.; Tsukamoto, S.; Kondo, K.; Iida, K. Dietary isoflavone daidzein promotes Tfam expression that increases mitochondrial biogenesis in C2C12 muscle cells. J. Nutr. Biochem. 2015, 26, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Erlangga, J.S.; Tsukamoto, S.; Sakamoto, Y.; Mabashi-Asazuma, H.; Iida, K. Daidzein promotes the expression of oxidative phosphorylation- and fatty acid oxidation-related genes via an estrogen-related receptor α pathway to decrease lipid accumulation in muscle cells. J. Nutr. Biochem. 2020, 77, 108315. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. J. Nutr. Biochem. 2014, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Orsatti, F.L.; Nahas, E.A.; Nahas-Neto, J.; Maesta, N.; Orsatti, C.L.; Fernandes, C.E. Effects of resistance training and soy isoflavone on body composition in postmenopausal women. Obstet. Gynecol. Int. 2010, 2010, 156037. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Godinez-Salas, E.T.; Sanchez-Tapia, M.; Torres-Villalobos, G.; Pichardo-Ontiveros, E.; Guizar-Heredia, R.; Arteaga-Sanchez, L.; Gamba, G.; Mojica-Espinosa, R.; Schcolnik-Cabrera, A.; et al. Genistein stimulates insulin sensitivity through gut microbiota reshaping and skeletal muscle AMPK activation in obese subjects. BMJ Open Diabetes Res. Care 2020, 8, e000948. [Google Scholar] [CrossRef] [Green Version]
- Kuryłowicz, A.; Cąkała-Jakimowicz, M.; Puzianowska-Kuźnicka, M. Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens. Nutrients 2020, 12, 582. [Google Scholar] [CrossRef] [Green Version]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv. Exp. Med. Biol. 2017, 1043, 227–256. [Google Scholar]
- Nogowski, L.; Maćkowiak, P.; Kandulska, K.; Szkudelski, T.; Nowak, K.W. Genistein-induced changes in lipid metabolism of ovariectomized rats. Ann. Nutr. Metab. 1998, 42, 360–366. [Google Scholar] [CrossRef]
- Li, H.; Kang, J.H.; Han, J.M.; Cho, M.H.; Chung, Y.J.; Park, K.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Jeong, T.S. Anti-obesity effects of soy leaf via regulation of adipogenic transcription factors and fat oxidation in diet-induced obese mice and 3T3-L1 adipocytes. J. Med. Food 2015, 18, 899–908. [Google Scholar] [CrossRef]
- Weigt, C.; Hertrampf, T.; Kluxen, F.M.; Flenker, U.; Hülsemann, F.; Fritzemeier, K.H.; Diel, P. Molecular effects of ER alpha- and beta-selective agonists on regulation of energy homeostasis in obese female Wistar rats. Mol. Cell. Endocrinol. 2013, 377, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.H.; Huang, S.Y.; Kung, C.W.; Chen, S.Y.; Chen, Y.F.; Cheng, P.Y.; Lam, K.K.; Lee, Y.M. Genistein ameliorated obesity accompanied with adipose tissue browning and attenuation of hepatic lipogenesis in ovariectomized rats with high-fat diet. J. Nutr. Biochem. 2019, 67, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Huang, C.; Luo, Q.; Liu, W.; Cheng, D.; Li, Y.; Xia, Y.; Li, C.; Tang, L.; Fang, J.; et al. Soy Isoflavones Ameliorate Fatty Acid Metabolism of Visceral Adipose Tissue by Increasing the AMPK Activity in Male Rats with Diet-Induced Obesity (DIO). Molecules 2019, 24, 2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Velpen, V.; Geelen, A.; Hollman, P.C.; Schouten, E.G.; van’t Veer, P.; Afman, L.A. Isoflavone supplement composition and equol producer status affect gene expression in adipose tissue: A double-blind, randomized, placebo-controlled crossover trial in postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 1269–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudelska, K.; Szkudelski, T.; Nogowski, L. Daidzein, coumestrol and zearalenone affect lipogenesis and lipolysis in rat adipocytes. Phytomedicine 2002, 9, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Genistein, a plant-derived isoflavone, counteracts the antilipolytic action of insulin in isolated rat adipocytes. J. Steroid Biochem. Mol. Biol. 2008, 109, 108–114. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, J.; Xiang, H.; Zeng, Y.Y.; Li, X.B.; Xiao, H.; Chen, D.Y.; Ma, R.L. Synthesis and biological evaluation of new flavonoid fatty acid esters with anti-adipogenic and enhancing glucose consumption activities. Bioorg. Med. Chem. 2011, 19, 3192–3203. [Google Scholar] [CrossRef]
- Nie, T.; Zhao, S.; Mao, L.; Yang, Y.; Sun, W.; Lin, X.; Liu, S.; Li, K.; Sun, Y.; Li, P.; et al. The natural compound, formononetin, extracted from Astragalus membranaceus increases adipocyte thermogenesis by modulating PPARγ activity. Br. J. Pharmacol. 2018, 175, 1439–1450. [Google Scholar] [CrossRef] [Green Version]
- Palacios-González, B.; Vargas-Castillo, A.; Velázquez-Villegas, L.A.; Vasquez-Reyes, S.; López, P.; Noriega, L.G.; Aleman, G.; Tovar-Palacio, C.; Torre-Villalvazo, I.; Yang, L.J.; et al. Genistein increases the thermogenic program of subcutaneous WAT and increases energy expenditure in mice. J. Nutr. Biochem. 2019, 68, 59–68. [Google Scholar] [CrossRef]
- Claussnitzer, M.; Skurk, T.; Hauner, H.; Daniel, H.; Rist, M.J. Effect of flavonoids on basal and insulin-stimulated 2-deoxyglucose uptake in adipocytes. Mol. Nutr. Food Res. 2011, 55, S26–S34. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Koźniewski, K. Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes. Molecules 2020, 25, 2224. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, S.H.; Ji, H.; Kim, J.E.; Yoo, R.; Kim, J.H.; Suk, S.; Huh, C.S.; Park, J.H.Y.; Heo, Y.S.; et al. Orobol, an enzyme-convertible product of genistein, exerts anti-obesity effects by targeting casein kinase 1 epsilon. Sci. Rep. 2019, 9, 8942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Gao, X.J.; Zhao, W.W.; Zhao, W.J.; Jiang, C.H.; Huang, F.; Kou, J.P.; Liu, B.L.; Liu, K. Opposite effects of genistein on the regulation of insulin-mediated glucose homeostasis in adipose tissue. Br. J. Pharmacol. 2013, 170, 328–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.J.; Yeh, Y.T.; Su, S.H.; Chang, K.L.; Shyu, H.W.; Chen, K.M.; Yeh, H. Biochanin a promotes osteogenic but inhibits adipogenic differentiation: Evidence with primary adipose-derived stem cells. Evid. Based Complement. Alternat. Med. 2013, 2013, 846039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadimi, D.; Taghi Goodarzi, M.; Ziamajidi, N.; Moradkhani, S. The effect of Biochanin A on the expression of Adiponectin in adipose tissue of Streptozotocin-Nicotinamide induced diabetic rats. Int. J. Med. Res. Health Sci. 2016, 5, 223–230. [Google Scholar]
- Sakamoto, Y.; Kanatsu, J.; Toh, M.; Naka, A.; Kondo, K.; Iida, K. The dietary isoflavone daidzein reduces expression of pro-inflammatory genes through PPARα/γ and JNK pathways in adipocyte and macrophage co-cultures. PLoS ONE 2016, 11, e0149676. [Google Scholar] [CrossRef]
- Matar, O.; Potier, L.; Abouleka, Y.; Hallot-Feron, M.; Fumeron, F.; Mohammedi, K.; Hadjadj, S.; Roussel, R.; Velho, G.; Marre, M. Relationship between renal capacity to reabsorb glucose and renal status in patients with diabetes. Diabetes Metab. 2020, 46, 488–495. [Google Scholar] [CrossRef]
- Jia, Q.; Yang, R.; Liu, X.F.; Ma, S.F.; Wang, L. Genistein attenuates renal fibrosis in streptozotocin-induced diabetic rats. Mol. Med. Rep. 2019, 19, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Lim, Y. Protective effect of short-term genistein supplementation on the early stage in diabetes-induced renal damage. Mediat. Inflamm. 2013, 2013, 510212. [Google Scholar] [CrossRef] [Green Version]
- Elmarakby, A.A.; Ibrahim, A.S.; Faulkner, J.; Mozaffari, M.S.; Liou, G.I.; Abdelsayed, R. Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vascul. Pharmacol. 2011, 55, 149–156. [Google Scholar] [CrossRef]
- Javanbakht, M.H.; Sadria, R.; Djalali, M.; Derakhshanian, H.; Hosseinzadeh, P.; Zarei, M.; Azizi, G.; Sedaghat, R.; Mirshafiey, A. Soy protein and genistein improves renal antioxidant status in experimental nephrotic syndrome. Nefrologia 2014, 34, 483–490. [Google Scholar] [PubMed]
- Laddha, A.P.; Kulkarni, Y.A. Daidzein Attenuates Kidney Damage in Diabetic Rats. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Katyal, T.; Garg, A.; Budhiraja, R.D. Combination of daidzein, hemin and bms182874 halts the progression of diabetes-induced experimental nephropathy. Endocr. Metab. Immune Disord. Drug Targets 2013, 13, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin attenuates kidney damage in type 2 diabetic rats. Life Sci. 2019, 219, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.R.; Tappenden, K.A.; Erdman, J.W., Jr. Altering dietary protein type and quantity reduces urinary albumin excretion without affecting plasma glucose concentrations in BKS.cg-m +Lepr db/+Lepr db (db/db) mice. J. Nutr. 2003, 133, 673–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, J.; Zhuang, K.; Jiang, X.; Huang, H.; Quan, S. Renoprotective Effect of Formononetin by Suppressing Smad3 Expression in Db/Db Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 3313–3324. [Google Scholar] [CrossRef]
- Do, M.H.; Hur, J.; Choi, J.; Kim, Y.; Park, H.Y.; Ha, S.K. Spatholobus suberectus Ameliorates Diabetes-Induced Renal Damage by Suppressing Advanced Glycation End Products in db/db Mice. Int. J. Mol. Sci. 2018, 19, 2774. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, S.R.; Tappenden, K.A.; Carson, L.; Jones, R.; Prabhudesai, M.; Marshall, W.P.; Erdman, J.W., Jr. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J. Nutr. 2004, 134, 1874–1880. [Google Scholar] [CrossRef] [Green Version]
- Jibani, M.M.; Bloodworth, L.L.; Foden, E.; Griffiths, K.D.; Galpin, O.P. Predominantly vegetarian diet in patients with incipient and early clinical diabetic nephropathy: Effects on albumin excretion rate and nutritional status. Diabet. Med. 1991, 8, 949–953. [Google Scholar] [CrossRef]
- Liu, Z.M.; Ho, S.C.; Chen, Y.M.; Tang, N.; Woo, J. Effect of whole soy and purified isoflavone daidzein on renal function—A 6-month randomized controlled trial in equol-producing postmenopausal women with prehypertension. Clin. Biochem. 2014, 47, 1250–1256. [Google Scholar] [CrossRef]
- Kim, W.; Egan, J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008, 60, 470–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, M.J.; Yang, H.J.; Park, S. Isoflavonoids and peptides from meju, long-term fermented soybeans, increase insulin sensitivity and exert insulinotropic effects in vitro. Nutrition 2011, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Ali, M.B.; Akash, M. Genistein enhances the secretion of glucagon-like peptide-1 (GLP-1) via downregulation of inflammatory responses. Biomed. Pharmacother. 2019, 112, 108670. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Shree, P.; Tripathi, Y.B. Active phytochemicals of Pueraria tuberosa for DPP-IV inhibition: In silico and experimental approach. J. Diabetes Metab. Disord. 2017, 21, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.S.; Lee, S.H. Genistein, a soy isoflavone, is a potent alpha-glucosidase inhibitor. FEBS Lett. 2001, 501, 84–86. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, L.; Flórez, A.B.; Guadamuro, L.; Mayo, B. Effect of Soy Isoflavones on Growth of Representative Bacterial Species from the Human Gut. Nutrients 2017, 9, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolca, S.; Possemiers, S.; Herregat, A.; Huybrechts, I.; Heyerick, A.; De Vriese, S.; Verbruggen, M.; Depypere, H.; De Keukeleire, D.; Bracke, M.; et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J. Nutr. 2007, 137, 2242–2246. [Google Scholar] [CrossRef] [Green Version]
- Guadamuro, L.; Delgado, S.; Redruello, B.; Flórez, A.B.; Suárez, A.; Martínez-Camblor, P.; Mayo, B. Equol status and changes in fecal microbiota in menopausal women receiving long-term treatment for menopause symptoms with a soy-isoflavone concentrate. Front. Microbiol. 2015, 6, 777. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Genser, L.; Aguanno, D.; Soula, H.A.; Dong, L.; Trystram, L.; Assmann, K.; Salem, J.E.; Vaillant, J.C.; Oppert, J.M.; Laugerette, F.; et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 2018, 246, 217–230. [Google Scholar] [CrossRef]
- López, P.; Sánchez, M.; Perez-Cruz, C.; Velázquez-Villegas, L.A.; Syeda, T.; Aguilar-López, M.; Rocha-Viggiano, A.K.; Del Carmen Silva-Lucero, M.; Torre-Villalvazo, I.; Noriega, L.G.; et al. Long-Term Genistein Consumption Modifies Gut Microbiota, Improving Glucose Metabolism, Metabolic Endotoxemia, and Cognitive Function in Mice Fed a High-Fat Diet. Mol. Nut. Food Res. 2018, 62, e1800313. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xu, J.; Lefever, D.E.; Glenn, T.C.; Nagy, T.; Guo, T.L. Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis. Toxicol. Appl. Pharmacol. 2017, 332, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Obici, S.; Feng, Z.; Karkanias, G.; Baskin, D.G.; Rossetti, L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002, 5, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiao, X.; Zhang, Q.; Zheng, J.; Li, M.; Deng, M. A Possible Mechanism: Genistein Improves Metabolism and Induces White Fat Browning Through Modulating Hypothalamic Expression of Ucn3, Depp, and Stc1. Front. Endocrinol. 2019, 10, 478. [Google Scholar] [CrossRef] [Green Version]
- Luo, T.; Miranda-Garcia, O.; Sasaki, G.; Wang, J.; Shay, N.F. Genistein and daidzein decrease food intake and body weight gain in mice, and alter LXR signaling in vivo and in vitro. Food Funct. 2018, 9, 6257–6267. [Google Scholar] [CrossRef]
- Fujitani, M.; Mizushige, T.; Bhattarai, K.; Iwahara, A.; Aida, R.; Kishida, T. The daidzein- and estradiol- induced anorectic action in CCK or leptin receptor deficiency rats. Biosci. Biotechnol. Biochem. 2015, 79, 1164–1171. [Google Scholar] [CrossRef]
- Pang, D.; Yang, C.; Luo, Q.; Li, C.; Liu, W.; Li, L.; Zou, Y.; Feng, B.; Chen, Z.; Huang, C. Soy isoflavones improve the oxidative stress induced hypothalamic inflammation and apoptosis in high fat diet-induced obese male mice through PGC1-alpha pathway. Aging 2020, 12, 8710–8727. [Google Scholar] [CrossRef]
- Weickert, M.O.; Reimann, M.; Otto, B.; Hall, W.L.; Vafeiadou, K.; Hallund, J.; Ferrari, M.; Talbot, D.; Branca, F.; Bügel, S.; et al. Soy isoflavones increase preprandial peptide YY (PYY), but have no effect on ghrelin and body weight in healthy postmenopausal women. J. Negat. Results Biomed. 2006, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Fujitani, M.; Mizushige, T.; Bhattarai, K.; Iwahara, A.; Aida, R.; Segawa, T.; Kishida, T. Dynamics of appetite-mediated gene expression in daidzein-fed female rats in the meal-feeding method. Biosci. Biotechnol. Biochem. 2015, 79, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Rivera, P.; Pérez-Martín, M.; Pavón, F.J.; Serrano, A.; Crespillo, A.; Cifuentes, M.; López-Ávalos, M.D.; Grondona, J.M.; Vida, M.; Fernández-Llebrez, P.; et al. Pharmacological administration of the isoflavone daidzein enhances cell proliferation and reduces high fat diet-induced apoptosis and gliosis in the rat hippocampus. PLoS ONE 2013, 8, e64750. [Google Scholar] [CrossRef]
- Thors, L.; Burston, J.J.; Alter, B.J.; McKinney, M.K.; Cravatt, B.F.; Ross, R.A.; Pertwee, R.G.; Gereau, R.W.; Wiley, J.L.; Fowler, C.J. Biochanin A, a naturally occurring inhibitor of fatty acid amide hydrolase. Br. J. Pharmacol. 2010, 160, 549–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.Y.; Daily, J.W.; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Pan, A.; Manson, J.E.; Willett, W.C.; Malik, V.; Rosner, B.; Giovannucci, E.; Hu, F.B.; Sun, Q. Consumption of soy foods and isoflavones and risk of type 2 diabetes: A pooled analysis of three US cohorts. Eur. J. Clin. Nutr. 2016, 70, 1381–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, K.P.; Kim, C.S.; Ahn, Y.; Park, S.J.; Kim, Y.J.; Park, J.K.; Lim, Y.K.; Yoo, K.Y.; Kim, S.S. Plasma isoflavone concentration is associated with decreased risk of type 2 diabetes in Korean women but not men: Results from the Korean Genome and Epidemiology Study. Diabetologia 2015, 58, 726–735. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Ryan, H.H.; Jones, E.; Simas, T.A.; Lichtenstein, A.H.; Sun, Q.; Hayman, L.L. Urinary isoflavone concentrations are inversely associated with cardiometabolic risk markers in pregnant U.S. women. J. Nutr. 2014, 144, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Talaei, M.; Lee, B.L.; Ong, C.N.; van Dam, R.M.; Yuan, J.M.; Koh, W.P.; Pan, A. Urine phyto-oestrogen metabolites are not significantly associated with risk of type 2 diabetes: The Singapore Chinese health study. Br. J. Nutr. 2016, 115, 1607–1615. [Google Scholar] [CrossRef] [Green Version]
- Villegas, R.; Gao, Y.T.; Yang, G.; Li, H.L.; Elasy, T.A.; Zheng, W.; Shu, X.O. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2008, 87, 162–167. [Google Scholar] [CrossRef]
- Yang, G.; Shu, X.O.; Jin, F.; Elasy, T.; Li, H.L.; Li, Q.; Huang, F.; Zhang, X.L.; Gao, Y.T.; Zheng, W. Soyfood consumption and risk of glycosuria: A cross-sectional study within the Shanghai Women’s Health Study. Eur. J. Clin. Nutr. 2004, 58, 615–620. [Google Scholar] [CrossRef]
- Pan, A.; Franco, O.H.; Ye, J.; Demark-Wahnefried, W.; Ye, X.; Yu, Z.; Li, H.; Lin, X. Soy protein intake has sex-specific effects on the risk of metabolic syndrome in middle-aged and elderly Chinese. J. Nutr. 2008, 138, 2413–2421. [Google Scholar] [CrossRef] [Green Version]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef]
- Mizoue, T.; Yamaji, T.; Tabata, S.; Yamaguchi, K.; Ogawa, S.; Mineshita, M.; Kono, S. Dietary patterns and glucose tolerance abnormalities in Japanese men. J. Nutr. 2006, 136, 1352–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rienks, J.; Barbaresko, J.; Nöthlings, U. Association of isoflavone biomarkers with risk of chronic disease and mortality: A systematic review and meta-analysis of observational studies. Nutr. Rev. 2017, 75, 616–641. [Google Scholar] [CrossRef] [PubMed]
- Rienks, J.; Barbaresko, J.; Oluwagbemigun, K.; Schmid, M.; Nöthlings, U. Polyphenol exposure and risk of type 2 diabetes: Dose-response meta-analyses and systematic review of prospective cohort studies. Am. J. Clin. Nutr. 2018, 108, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ruan, W.; Peng, Y.; Wang, D. Soy and the risk of type 2 diabetes mellitus: A systematic review and meta-analysis of observational studies. Diabetes Res. Clin. Pract. 2018, 137, 190–199. [Google Scholar] [CrossRef]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine (Baltimore) 2018, 97, e0686. [Google Scholar] [CrossRef]
- Guo, X.F.; Ruan, Y.; Li, Z.H.; Li, D. Flavonoid subclasses and type 2 diabetes mellitus risk: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 2850–2862. [Google Scholar] [CrossRef]
- Tang, J.; Wan, Y.; Zhao, M.; Zhong, H.; Zheng, J.S.; Feng, F. Legume and soy intake and risk of type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2020, 111, 677–688. [Google Scholar] [CrossRef]
- Tsai, A.C.; Vinik, A.I.; Lasichak, A.; Lo, G.S. Effects of soy polysaccharide on postprandial plasma glucose, insulin, glucagon, pancreatic polypeptide, somatostatin, and triglyceride in obese diabetic patients. Am. J. Clin. Nutr. 1987, 45, 596–601. [Google Scholar] [CrossRef]
- Jayagopal, V.; Albertazzi, P.; Kilpatrick, E.S.; Howarth, E.M.; Jennings, P.E.; Hepburn, D.A.; Atkin, S.L. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care 2002, 25, 1709–1714. [Google Scholar] [CrossRef] [Green Version]
- Curtis, P.J.; Sampson, M.; Potter, J.; Dhatariya, K.; Kroon, P.A.; Cassidy, A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: A 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012, 35, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Squadrito, F.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; D’Anna, R.; Arcoraci, V.; Burnett, B.P.; Minutoli, L.; et al. Genistein in the metabolic syndrome: Results of a randomized clinical trial. J. Clin. Endocrinol. Metab. 2013, 98, 3366–3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathyapalan, T.; Rigby, A.S.; Bhasin, S.; Thatcher, N.J.; Kilpatrick, E.S.; Atkin, S.L. Effect of Soy in Men with Type 2 Diabetes Mellitus and Subclinical Hypogonadism: A Randomized Controlled Study. J. Clin. Endocrinol. Metab. 2017, 102, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braxas, H.; Rafraf, M.; Karimi Hasanabad, S.; Asghari Jafarabadi, M. Effectiveness of Genistein Supplementation on Metabolic Factors and Antioxidant Status in Postmenopausal Women with Type 2 Diabetes Mellitus. Can. J. Diabetes 2019, 43, 490–497. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Jayagopal, V.; Kilpatrick, E.S.; Chapman, T.; Atkin, S.L. Effects of isoflavone dietary supplementation on cardiovascular risk factors in type 2 diabetes. Diabetes Care 2007, 30, 1871–1873. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.M.; Chen, Y.M.; Ho, S.C.; Ho, Y.P.; Woo, J. Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: A 6-mo double-blind, randomized, placebo-controlled trial in postmenopausal Chinese women with prediabetes or untreated early diabetes. Am. J. Clin. Nutr. 2010, 91, 1394–1401. [Google Scholar] [CrossRef] [Green Version]
- Hermansen, K.; Søndergaard, M.; Høie, L.; Carstensen, M.; Brock, B. Beneficial effects of a soy-based dietary supplement on lipid levels and cardiovascular risk markers in type 2 diabetic subjects. Diabetes Care 2001, 24, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Vafeiadou, K.; Hall, W.L.; Williams, C.M. Does genotype and equol-production status affect response to isoflavones? Data from a pan-European study on the effects of isoflavones on cardiovascular risk markers in post-menopausal women. Proc. Nutr. Soc. 2006, 65, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Charles, C.; Yuskavage, J.; Carlson, O.; John, M.; Tagalicud, A.S.; Maggio, M.; Muller, D.C.; Egan, J.; Basaria, S. Effects of high-dose isoflavones on metabolic and inflammatory markers in healthy postmenopausal women. Menopause 2009, 16, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Gobert, C.P.; Pipe, E.A.; Capes, S.E.; Darlington, G.A.; Lampe, J.W.; Duncan, A.M. Soya protein does not affect glycaemic control in adults with type 2 diabetes. Br. J. Nutr. 2010, 103, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.M.; Chen, Y.M.; Ho, S.C. Effects of soy intake on glycemic control: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2011, 93, 1092–1101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.B.; Chen, W.H.; Guo, J.J.; Fu, Z.H.; Yi, C.; Zhang, M.; Na, X.L. Soy isoflavone supplementation could reduce body weight and improve glucose metabolism in non-Asian postmenopausal women-a meta-analysis. Nutrition 2013, 29, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Glisic, M.; Kastrati, N.; Musa, J.; Milic, J.; Asllanaj, E.; Fernandez, E.P.; Nano, J.; Rosales, C.O.; Amiri, M.; Kraja, B.; et al. Phytoestrogen supplementation and body composition in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2018, 115, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Soltanipour, S.; Hasandokht, T.; Soleimani, R.; Mahdavi-Roshan, M.; Jalali, M.M. Systematic Review and Meta-Analysis of the Effects of Soy on Glucose Metabolism in Patients with Type 2 Diabetes. Rev. Diabet. Stud. 2019, 15, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Genistein | Daidzein | Formononetin | Biochanin A | Molecular Mechanism | Experimental Model | Reference | |
|---|---|---|---|---|---|---|---|
| β-cells | ↑insulin secretion | ↑insulin secretion | ↓NF-κB ↓ERK-1/2 | RIN cells | [20] [33] | ||
| ↑proliferation | STZ rats | [38] | |||||
| ↓DNA fragmentation | ↑ERβ | human β-cells | [31] | ||||
| ↑↓apoptosis * | ↑↓ERK1/2 | rodent β-cells | [32] | ||||
| ↑proliferation | ↓apoptosis | ↑proliferation | ↑AMPK | INS-1 cells | [33,42,47] | ||
| ↓apoptosis | ↓PKA ↑PP2A | INS-1 cells ZDF rats | [43] | ||||
| ↑insulin secretion ↓apoptosis | ↑insulin secretion | ↑AMPK ↑calcineurin | INS-1 cells mouse pancreatic islets STZ/HFD mice db/db mice | [35,36] [34] [37,38,39] [40] | |||
| ↓inflammation | ↑insulin secretion | ↑insulin secretion* | ↑SIRT1 | alloxan-induced diabetic mice ovariectomized rats STZ rats | [44] [41] [47,48] | ||
| ↓apoptosis | ↓NF-κB | INS-1 cells STZ rats | [46] [45] | ||||
| α-cells | ↓glucagon secretion | ? | geese broilers db/db mice | [49] [50] [40] | |||
| =glucagon secretion | alloxan-induced diabetic mice | [44] | |||||
| liver | ↓gluconeogenesis ↑fatty acid oxidation | ↓gluconeogenesis ↑fatty acid oxidation | ↑methylation | NOD mice HFD diabetic mice monkeys db/db mice insulin resistant rats | [53] [54] [24] [40] [56] | ||
| ↓glycogenolysis | STZ rats | [45] | |||||
| ↑antioxidant activity | ↑antioxidant activity | STZ rats and mice | [37,55] | ||||
| ↓gluconeogenesis | HFD diabetic mice | [57] | |||||
| =steatosis | =steatosis | ZDF rats | [59] | ||||
| ↓steatosis | ↓steatosis | ↑PPARα | HFD diabetic mice | [66] | |||
| ↓steatosis | ↓steatosis | ↓steatosis | ↓steatosis | ↑AMPK | HFD diabetic mice | [60,63,64,66] | |
| ↓steatosis | ↓COX-1 | HFD diabetic rodents db/db mice | [19,56,58,65] [62] | ||||
| ↓steatosis | Buffalo Rat liver | [61] | |||||
| muscle | ↑proliferation | ↓miR-222 | C2C12 myotubes | [27] | |||
| ↑mitochondrial biogenesis | ↑mitochondrial biogenesis ↑fatty acid oxidation | ↑SIRT1 ↑NrF ↑ERα | [74] [75] | ||||
| ↑fatty acid oxidation | ↑insulin sensitivity | ↑AMPK ↑ PPARδ ↑GLUT4 | C2C12 myotubes ZDF rats db/db mice | [71] [76] | |||
| = insulin sensitivity | = insulin sensitivity | =PPARγ =PPARα | ZDF rats cynomolgus monkeys | [72] [73] | |||
| ↑insulin sensitivity | ↑insulin sensitivity | ↑IRS1 ↑GLUT4 | HFD diabetic mice | [57] | |||
| ↑insulin sensitivity | MetS patients | [77,78] | |||||
| adipose tissue | ↓lipid synthesis | ↑lipolysis ↑thermogenesis | ↑PPARγ | 3T3-L1 preadipocytes ovariectomized rats C57BL/6 obese mice obese Wistar rats | [82,95] [81,84] [63,90,91] [92] | ||
| ↓lipid synthesis | ↓lipid synthesis | ↑AMPK | 3T3-L1 preadipocytes HFD rodents human AT | [85] [54] [86] | |||
| ↑lipolysis | ↑lipolysis | ↓cAMP PDE | rat adipocytes C57BL/6 obese mice | [87,88] [63] | |||
| ↑lipolysis | ↓miR-222 | 3T3-L1 preadipocytes HFD mice | [28] | ||||
| ↓inflammation | ↓NF-κB | 3T3-L1 preadipocytes | [94] | ||||
| ↓inflammation | ↑PPARα ↑PPARγ | C57BL/6 obese mice 3T3-L1 preadipocytes human AT | [63] [98] [86] | ||||
| ↓inflammation ↑adiponectin | ↓PPARγ | rat adipocytes STZ rats | [96] [97] | ||||
| kidney | ↓oxidative stress ↓fibrosis ↓inflammation | ↓oxidative stress ↓fibrosis | ↑NrF ↓TGF‑β ↓NF-κB | STZ rodents | [100,101,102,104,105] | ||
| ↓albuminuria ↑GFR | ↓albuminuria ↑GFR | ↓albuminuria ↑GFR | ↓smad3 | db/db mice | [107,108,109] | ||
| ↓albuminuria ↑GFR | ↓albuminuria ↑GFR | T2D patients | [110,111] | ||||
| =albuminuria =GFR | T2D patients | [112] | |||||
| ↑GFR | ↑SIRT1 | STZ rats | [106] | ||||
| gastrointestinal tract | ↑GLP-1 | ↑GLP-1 | ↓NF-κB | enteroendocrine cells alloxan-induced diabetic rats STZ rats | [114,115] [115] [116] | ||
| ↓inflammation | ↓inflammation | modulation of gut microbiota | HFD mice NOD mice | [121,122,123] [124] | |||
| brain | ↑insulin sensitivity ↓oxidative stress ↓inflammation | ↑insulin sensitivity ↓oxidative stress ↓inflammation | modulation of hypothalamic gene expression | HFD mice ObRb(-)rats | [126,127] [128] | ||
| ↓appetite | ↓appetite | peptide YY | healthy women | [130] | |||
| ↑appetite | Wistar & Sprague-Dawley rats | [132,133] |
| Study Design | Studied Group | Measurements | Intervention | Outcomes | Reference |
|---|---|---|---|---|---|
| epidemiological studies | |||||
| NHS NHSII HPFS prospective | 163,457 individuals (142176 F) 9181 T2D cases (8439 F) | soy and isoflavones intake assessed with a validated FFQ | not applicable | inverse association between isoflavones intake and T2D risk | [135] |
| Korean Genome and Epidemiology Study case-control | 698 healthy controls (317 F) 693 T2D patients (316 F) | plasma levels of isoflavones, genistein, daidzein, equol, glyctein | not applicable | inverse association between plasma genistein level and T2D risk | [136] |
| NHANES 2001–2010 cross-sectional | 299 healthy pregnant women | urinary concentrations of total isoflavones metabolites | not applicable | inverse association between urinary concentrations of total isoflavone metabolites with FPG, insulin, HOMA-IR | [137] |
| Singapore Chinese Health Study cross-sectional | 564 healthy controls (329 F, 192 postmenopausal) 564 T2D patients (329 F, 199 postmenopausal) | urinary concentrations of total isoflavones metabolites | not applicable | inverse association between daidzein and genistein intake and T2D risk | [138] |
| Shanghai Women’s Health Study prospective | 64 227 pre- and postmenopausal women 1608 T2D cases | legumes intake assessed with a validated FFQ (follow-up survey after 2–3 years) | not applicable | inverse association between soybeans intake and T2D incidence | [139] |
| Shanghai Women’s Health Study | 39 385 postmenopausal women 323 T2D cases | soy products intake assessed with a validated FFQ | not applicable | inverse association between soy products intake and risk of glycosuria | [140] |
| Nutrition and Health of Aging Population in China project cross-sectional | 2811 individuals (1638 F) | soy protein intake assessed with a validated FFQ | not applicable | positive association between soy protein intake with hyperglycemia in men | [141] |
| meta-analyses of epidemiological studies | |||||
| NHS NHSII Singapore Chinese Health Study Korean Genome and Epidemiology Study | 144,695 individuals 9696 T2D cases | soy and isoflavones intake assessed with a validated FFQ; urine/plasma levels of isoflavones, genistein, daidzein, equol, glyctein | not applicable | inverse association between isoflavones, genistein, and daidzein and T2D incidence | [144] |
| 6 prospective cohort studies | 299,667 individuals 24,469 T2D cases | isoflavones intake assessed with a validated FFQ | not applicable | inverse association between isoflavones intake and T2D incidence | [145] |
| 6 prospective cohort studies 2 cross-sectional studies | 1,901,230 individuals 7589 T2D cases | soy products and isoflavones intake assessed with a validated FFQ | not applicable | inverse association between isoflavones intake and T2D incidence | [146] |
| 8 prospective cohort studies | 312,015 individuals 19,953 T2D cases | total flavonoids, anthocyanidins, flavan-3-ols, flavolons and isoflavones intake assessed with a validated FFQ | not applicable | inverse and dose-dependent association between total flavonoids, anthocyanidins, flavan-3-ols, flavolons and isoflavones intake and T2D incidence | [147] |
| 9 prospective cohort studies | 172,058 individuals 16,910 T2D cases | flavanols, flavonols, flavan-3-ols and isoflavones intake assessed with a validated FFQ | not applicable | inverse and dose-dependent association between flavanols, flavonols, flavan-3-ols and isoflavones intake and T2D incidence | [148] |
| 15 prospective cohort studies | 565,810 individuals 32,093 T2D cases | legumes, total soy, soy milk, tofu, soy proteins and soy isoflavones intake assessed with a validated FFQ | not applicable | inverse association between tofu, soy proteins and soy isoflavones intake and T2D incidence | [149] |
| interventional studies | |||||
| RCT 2 arms cross-over (3-week washout period separating the placebo and active phases 12 weeks each) | 32 postmenopausal women with T2D | FPG fasting insulin HOMA-IR HbA1c TC HDL-C LDL-C | 132 mg isoflavones/day | ↓fasting insulin ↓HOMA-IR ↓HbA1c ↓TC ↓LDL-C ↓TC/HDL-C ↓fT4 | [152] |
| RCT 2 arms parallel (1 year) | 93 postmenopausal women with T2D | FPG fasting insulin HOMA-IR TC HDL-C LDL-C | 850 mg flavan-3-ols/day 100 mg isoflavones/day | ↓HOMA-IR ↓fasting insulin ↓HDL-C ↓LDL-C | [151] |
| RCT 2 arms parallel (1 year) | 120 postmenopausal women with MetS | FPG fasting insulin HOMA-IR TC HDL-C LDL-C TG adiponectin | 54 mg genistein/day | ↓FPG ↓fasting insulin ↓HOMA-IR ↓TC ↑HDL-C ↓LDL-C =TG ↑adiponectin | [153] |
| RCT 2 arms parallel (3 months) | 200 men with T2D and subclinical hypogonadism | testosterone TSH fT4 HbA1c HOMA-IR TG CRP | 66 mg isoflavones/day | =testosterone ↑TSH ↓fT4 ↓HbA1c ↓HOMA-IR ↓TG ↓CRP | [154] |
| RCT 2 arms parallel (12 weeks) | 54 postmenopausal women with T2D | FPG fasting insulin HOMA-IR HDL-C TG | 108 mg genistein/day | ↓FPG ↓fasting insulin ↓HOMA-IR ↑HDL-C ↓TG | [155] |
| RCT 2 arms cross-over (4-week washout period separating the placebo and active phases 12 weeks each) | 26 postmenopausal women with T2D | FPG fasting insulin HOMA-IR HbA1c TC TG HDL-C LDL-C CRP | 132 mg isoflavones/day | =FPG =fasting insulin =HOMA-IR =HbA1c =TC =TG =HDL-C =LDL-C =CRP | [156] |
| RCT 2 arms cross-over (3-week washout period separating the placebo and active phases 6 weeks each) | 14 male, 6 female T2D patients | FPG fasting insulin postprandial glucose postprandial insulin TC HDL-C LDL-C TG apoB100 homocysteine | 165 mg isoflavones/day | =FPG =fasting insulin =postprandial glucose =postprandial insulin ↓TC ↓LDL-C ↓LDL-C/HDL-C ↓apoB100 ↓TG ↓homocysteine | [157] |
| RCT 3 arms parallel (6 months) | 180 postmenopausal women with prediabetes or early untreated T2D | FPG 2h post-load glucose insulin HOMA-IR HbA1c | 15 g soy protein + 100 mg isoflavones, 15 g milk protein +100 mg isoflavones, 15 g milk protein | =FPG =2h post-load glucose =fasting insulin =HOMA-IR =HbA1c | [158] |
| RCT 2 arms cross-over (3-week washout period separating the placebo and active phases 6 weeks each) | 117 healthy post-menopausal women | FPG fasting insulin HOMA-IR HbA1c TC TG HDL-C LDL-C CRP | 50 mg isoflavones/day | =FPG =fasting insulin =HOMA-IR =HbA1c =TC =TG =HDL-C =LDL-C ↓CRP | [159] |
| RCT 2 arms parallel (12 weeks) | 75 healthy postmenopausal women | FPG fasting insulin HOMA-IR adiponectin resistin leptin | 160 mg of total isoflavones (64 mg genistein, 63 mg daidzein, and 34 mg glycitein) | =FPG =fasting insulin =HOMA-IR ↑adiponectin =resistin =leptin | [160] |
| RCT 2 arms cross-over (4-week washout period separating the placebo and active phases 57 days each) | 29 individuals with T2D (men and postmenopausal women) | FPG postprandial glucose fasting insulin postprandial insulin HOMA-IR | 88 mg of total isoflavones (65% genistein, 31% daidzein and 4% glycitein) | =FPG =postprandial glucose =fasting insulin =postprandial insulin =HOMA-IR | [161] |
| Meta-analyses of interventional studies | |||||
| 24 RCTs: 18 on women, (14 on postmenopausal) 6 on T2D patients 15 parallel design 9 cross-over | 1518 subjects of different nationality, sexes, ages, and glycemic status | FPG fasting insulin HOMA-IR | soy-protein: 0–40 g/d, isoflavone content: 36–132 mg/d, time: 4–52 weeks | whole group: =FPG =fasting insulin subgroup analysis: whole soy foods ↓FPG | [162] |
| 12 RCTs 4 on normal-weight women 8 on obese women | non-Asian postmenopausal women 601 intervention arm for FPG 581 placebo arm for FPG 582 intervention arm for fasting insulin 561 placebo arm for fasting insulin | FPG fasting insulin | isoflavone content: 40 to 160 mg time: 8–52 weeks | ↓FPG ↓fasting insulin | [163] |
| 23 RCTs: 19 on healthy women 4 on T2D women | 1880 postmenopausal women of different nationality: 1130 intervention arm 750 placebo arm | BMI WHR | soy-protein:25–40 g/d, isoflavone content: 60–270 mg/d, time: 8–48 weeks | ↑BMI in women with pre-existing: - prediabetes, - T2D, - prehypertension, - hyperlipidemia | [164] |
| 16 RCTs: 6 parallel 10 cross-over | 471 T2D patients (315 F) 43% of women postmenopausal | FPG fasting insulin HbA1c | soy-protein: 0.8–50 g/d, isoflavone content: 32–165 mg/d, time: 4 weeks–4 years | =FPG in 14 RCTs =insulin levels in 11 RCTs =HbA1c in 13 RCTs | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuryłowicz, A. The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment—A Narrative Review. Int. J. Mol. Sci. 2021, 22, 218. https://doi.org/10.3390/ijms22010218
Kuryłowicz A. The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment—A Narrative Review. International Journal of Molecular Sciences. 2021; 22(1):218. https://doi.org/10.3390/ijms22010218
Chicago/Turabian StyleKuryłowicz, Alina. 2021. "The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment—A Narrative Review" International Journal of Molecular Sciences 22, no. 1: 218. https://doi.org/10.3390/ijms22010218