Angiopoietin-2 Promotes Inflammatory Activation in Monocytes of Systemic Sclerosis Patients
Abstract
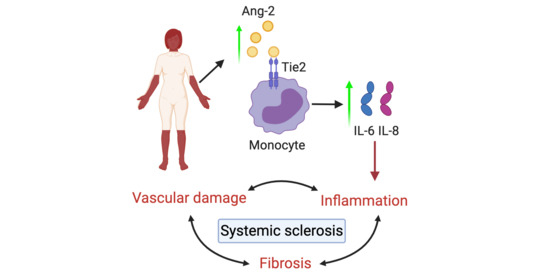
:1. Introduction
2. Results
2.1. Ang-2 Levels Are Elevated in the Serum of SSc Patients
2.2. Monocyte-Ang-2 Stimulation Induces IL-6 and IL-8 Secretion in SSc Patients
3. Discussion
4. Materials and Methods
4.1. Patients and Controls
4.2. Monocyte Isolation
4.3. Dermal Fibroblast Isolation and Culture
4.4. Human Pulmonary Arterial Endothelial Cells Cell Culture
4.5. Cell Stimulation
4.6. Flow Cytometry
4.7. RT-PCR and Quantitative (q)PCR
4.8. Ang-2 Expression from Profiling Data
4.9. Measurement of Cytokine Production
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef]
- Van Bon, L.; Cossu, M.; Radstake, T.R.D.J. An update on an immune system that goes awry in systemic sclerosis. Curr. Opin. Rheumatol. 2011, 23, 505–510. [Google Scholar] [CrossRef]
- Worrell, J.C.; O’Reilly, S. Bi-directional communication: Conversations between fibroblasts and immune cells in systemic sclerosis. J. Autoimmun. 2020, 113, 102526. [Google Scholar] [CrossRef]
- Carvalheiro, T.; Horta, S.; van Roon, J.A.G.; Santiago, M.; Salvador, M.J.; Trindade, H.; Radstake, T.R.D.J.; da Silva, J.A.P.; Paiva, A. Increased frequencies of circulating CXCL10-, CXCL8- and CCL4-producing monocytes and Siglec-3-expressing myeloid dendritic cells in systemic sclerosis patients. Inflamm. Res. 2018, 67, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Ciechomska, M.; Huigens, C.A.; Hugle, T.; Stanly, T.; Gessner, A.; Griffiths, B.; Radstake, T.R.D.J.; Hambleton, S.; O’Reilly, S.; van Laar, J.M. Toll-like receptor-mediated, enhanced production of profibrotic TIMP-1 in monocytes from patients with systemic sclerosis: Role of serum factors. Ann. Rheum. Dis. 2013, 72, 1382–1389. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, S.; Cant, R.; Ciechomska, M.; van Laar, J.M. Interleukin-6: A new therapeutic target in systemic sclerosis? Clin. Transl. Immunol. 2013, 2, e4. [Google Scholar] [CrossRef]
- Ciechomska, M.; Cant, R.; Finnigan, J.; van Laar, J.M.; O’Reilly, S. Role of toll-like receptors in systemic sclerosis. Expert Rev. Mol. Med. 2013, 15, e9. [Google Scholar] [CrossRef]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef]
- Venneri, M.A.; De Palma, M.; Ponzoni, M.; Pucci, F.; Scielzo, C.; Zonari, E.; Mazzieri, R.; Doglioni, C.; Naldini, L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 2007, 109, 5276–5285. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, C.; Tazzyman, S.; Webster, S.; Lewis, C.E. Expression of Tie-2 by Human Monocytes and Their Responses to Angiopoietin-2. J. Immunol. 2007, 178, 7405–7411. [Google Scholar] [CrossRef] [Green Version]
- Krausz, S.; Garcia, S.; Ambarus, C.A.; De Launay, D.; Foster, M.; Naiman, B.; Iverson, W.; Connor, J.R.; Sleeman, M.A.; Coyle, A.J.; et al. Angiopoietin-2 promotes inflammatory activation of human macrophages and is essential for murine experimental arthritis. Ann. Rheum. Dis. 2012, 71, 1402–1410. [Google Scholar] [CrossRef]
- García, S.; Krausz, S.; Ambarus, C.A.; Fernández, B.M.; Hartkamp, L.M.; Van Es, I.E.; Hamann, J.; Baeten, D.L.; Tak, P.P.; Reedquist, K.A. Tie2 signaling cooperates with TNF to promote the pro-inflammatory activation of human macrophages independently of macrophage functional phenotype. PLoS ONE 2014, 9, e82088. [Google Scholar] [CrossRef]
- Kabala, P.A.; Malvar-Fernández, B.; Lopes, A.P.; Carvalheiro, T.; Hartgring, S.A.Y.; Tang, M.W.; Conde, C.; Baeten, D.L.; Sleeman, M.; Tak, P.P.; et al. Promotion of macrophage activation by Tie2 in the context of the inflamed synovia of rheumatoid arthritis and psoriatic arthritis patients. Rheumatology (U. K.) 2020, 59, 426–438. [Google Scholar] [CrossRef] [Green Version]
- Michalska-Jakubus, M.; Kowal-Bielecka, O.; Chodorowska, G.; Bielecki, M.; Krasowska, D. Angiopoietins-1 and -2 are differentially expressed in the sera of patients with systemic sclerosis: High angiopoietin-2 levels are associated with greater severity and higher activity of the disease. Rheumatology 2011, 50, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Gerlicz, Z.; Dziankowska-Bartkowiak, B.; Dziankowska-Zaborszczyk, E.; Sysa-Jedrzejowska, A. Disturbed balance between serum levels of receptor tyrosine Kinases Tie-1, Tie-2 and angiopoietins in systemic sclerosis. Dermatology 2014, 228, 233–239. [Google Scholar] [CrossRef]
- Cossu, M.; Andracco, R.; Santaniello, A.; Marchini, M.; Severino, A.; Caronni, M.; Radstake, T.; Beretta, L. Serum levels of vascular dysfunction markers reflect disease severity and stage in systemic sclerosis patients. Rheumatology 2016, 55, 1112–1116. [Google Scholar] [CrossRef] [Green Version]
- Moritz, F.; Schniering, J.; Distler, J.H.W.; Gay, R.E.; Gay, S.; Distler, O.; Maurer, B. Tie2 as a novel key factor of microangiopathy in systemic sclerosis. Arthritis Res. Ther. 2017, 19, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Michalska-Jakubus, M.; Cutolo, M.; Smith, V.; Krasowska, D. Imbalanced serum levels of Ang1, Ang2 and VEGF in systemic sclerosis: Integrated effects on microvascular reactivity. Microvasc. Res. 2019, 125, 103881. [Google Scholar] [CrossRef] [Green Version]
- Eloranta, M.-L.; Franck-Larsson, K.; Lövgren, T.; Kalamajski, S.; Rönnblom, A.; Rubin, K.; Alm, G.V.; Rönnblom, L. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann. Rheum. Dis. 2010, 69, 1396–1402. [Google Scholar] [CrossRef]
- Khan, K.; Xu, S.; Nihtyanova, S.; Derrett-Smith, E.; Abraham, D.; Denton, C.P.; Ong, V.H. Clinical and pathological significance of interleukin 6 overexpression in systemic sclerosis. Ann. Rheum. Dis. 2012, 71, 1235–1242. [Google Scholar] [CrossRef]
- O’Reilly, S.; Ciechomska, M.; Cant, R.; Van Laar, J.M. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via gremlin protein. J. Biol. Chem. 2014, 289, 9952–9960. [Google Scholar] [CrossRef] [Green Version]
- King, J.; Abraham, D.; Stratton, R. Chemokines in systemic sclerosis. Immunol. Lett. 2018, 195, 68–75. [Google Scholar] [CrossRef]
- Kim, M.; Allen, B.; Korhonen, E.A.; Nitschké, M.; Yang, H.W.; Baluk, P.; Saharinen, P.; Alitalo, K.; Daly, C.; Thurston, G.; et al. Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J. Clin. Investig. 2016, 126, 3511–3525. [Google Scholar] [CrossRef]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An american college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [Green Version]




| Patient Characteristics | ELISA Angiopoietins | Dermal Fibroblasts | Functional Assays | |||
|---|---|---|---|---|---|---|
| HC (n = 20) | SSc (n = 27) | HC (n = 6) | SSc (n = 5) | HC (n = 24) | SSc (n = 27) | |
| Age (years) | 44 (37–50) | 64 (49–68) | 40 (40–52) | 42 (36–45) | 50 (42–57) | 53 (42–66) |
| Female: n (%) | 17 (85) | 21 (78) | 6 (100) | 6 (60) | 18 (75) | 19 (70) |
| Disease duration (years) | 9 (2–14) | 2 (1–3) | 8 (3–15) | |||
| Limited cutaneous SSc | 15 (55) | 1 (20) | 11 (40) | |||
| Diffuse cutaneous SSc | 12 (45) | 4 (80) | 16 (60) | |||
| ANA positive: n (%) | 26 (96) | 4 (80) | 26 (96) | |||
| ACA positive: n (%) | 9 (33) | 0 (0) | 7 (26) | |||
| Scl70 positive: n (%) | 8 (27) | 1 (20) | 13 (48) | |||
| mRSS | 4 (2–9) | 7 (7–8) | 7 (4–13) | |||
| FVC | 104 (90–119) | N.D. | 91 (83–98) | |||
| ILD: n (%) | 7 (26) | 1 (20) | 6 (22) | |||
| DMARDs: n (%) | 18 (66) | 4 (80) | 15 (55) | |||
| Biologicals: n (%) | 0 (0) | 0 (0) | 1 (4) | |||
| Cell Population | Gating Strategy |
|---|---|
| Monocytes | HLA-DR+ CD11c+ CD14+ |
| Classical monocytes | HLA-DR+ CD11c+ CD14+ CD16− |
| Intermediate monocytes | HLA-DR+ CD11c+ CD14+ CD16+ |
| Non-classical monocytes | HLA-DR+ CD11c+ CD14− CD16+ |
| Gene | Primer Forward | Primer Reverse |
|---|---|---|
| ANG2 | 5′TCAGTGGCTAATGAAGCTTGAGA3′ | 5′CCGCTGTTTGGTTCAACAGG3′ |
| GAPDH | 5′GCCAGCCGAGCCACATC3′ | 5′TGACCAGGCGCCCAATAC3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalheiro, T.; Lopes, A.P.; van der Kroef, M.; Malvar-Fernandez, B.; Rafael-Vidal, C.; Hinrichs, A.C.; Servaas, N.H.; Bonte-Mineur, F.; Kok, M.R.; Beretta, L.; et al. Angiopoietin-2 Promotes Inflammatory Activation in Monocytes of Systemic Sclerosis Patients. Int. J. Mol. Sci. 2020, 21, 9544. https://doi.org/10.3390/ijms21249544
Carvalheiro T, Lopes AP, van der Kroef M, Malvar-Fernandez B, Rafael-Vidal C, Hinrichs AC, Servaas NH, Bonte-Mineur F, Kok MR, Beretta L, et al. Angiopoietin-2 Promotes Inflammatory Activation in Monocytes of Systemic Sclerosis Patients. International Journal of Molecular Sciences. 2020; 21(24):9544. https://doi.org/10.3390/ijms21249544
Chicago/Turabian StyleCarvalheiro, Tiago, Ana P. Lopes, Maarten van der Kroef, Beatriz Malvar-Fernandez, Carlos Rafael-Vidal, Anneline C. Hinrichs, Nila H. Servaas, Femke Bonte-Mineur, Marc R. Kok, Lorenzo Beretta, and et al. 2020. "Angiopoietin-2 Promotes Inflammatory Activation in Monocytes of Systemic Sclerosis Patients" International Journal of Molecular Sciences 21, no. 24: 9544. https://doi.org/10.3390/ijms21249544