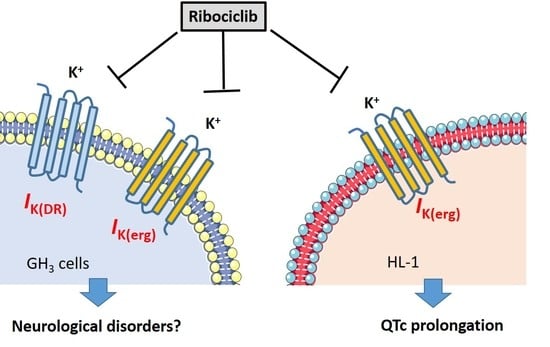
Characterization of the Synergistic Inhibition of IK(erg) and IK(DR) by Ribociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs, Chemicals, and Solutions Prepared for This Study
2.2. Cell Preparations
2.3. Electrophysiological Measurements
2.4. Data Recordings
2.5. Data Analyses
2.6. Statistical Analyses
3. Results
3.1. Effect of RIB on Erg-Mediated K+ Current (IK(erg)) Identified in Pituitary GH3 Cells
3.2. Effect of RIB on the Mean Current–Voltage (I–V) Relationship of IK(erg)
3.3. Effect of RIB on the Voltage Hysteresis of IK(erg) Elicited during an Isosceles-Triangular Ramp Pulse
3.4. Effect of RIB on Delayed-Rectifier K+ Current (IK(DR)) Recorded from GH3 Cells
3.5. Effect of RIB on 10-s Long Depolarization-Elicited IK(DR)
3.6. Effect of RIB on M-Type K+ Current (IK(M)) in GH3 Cells
3.7. Lack of RIB Effect on the Perturbation of Hyperpolarization-Activated Cation Current (Ih) in GH3 Cells
3.8. Effect of RIB on IK(erg) Identified in HL-1 Atrial Cardiomyocytes
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Roskoski, R. Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacol. Res. 2019, 139, 471–488. [Google Scholar] [CrossRef]
- Konecny, G.E. Cyclin-dependent kinase pathways as targets for women’s cancer treatment. Curr. Opin. Obstet. Gynecol. 2016, 28, 42–48. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Obinata, D.; Sandhu, S.; Selth, L.A.; Wong, S.Q.; Porter, L.H.; Lister, N.; Pook, D.; Pezaro, C.J.; Goode, D.L.; et al. Patient-derived Models of Abiraterone- and Enzalutamide-resistant Prostate Cancer Reveal Sensitivity to Ribosome-directed Therapy. Eur. Urol. 2018, 74, 562–572. [Google Scholar] [CrossRef]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- Lee, K.A.; Shepherd, S.T.; Johnston, S.R.D. Abemaciclib, a potent cyclin-dependent kinase 4 and 6 inhibitor, for treatment of ER-positive metastatic breast cancer. Futur. Oncol. 2019, 15, 3309–3326. [Google Scholar] [CrossRef]
- Bai, F.; Pei, X.-H.; Nishikawa, T.; Smith, M.D.; Xiong, Y. p18Ink4c, but not p27Kip1, Collaborates with Men1 To Suppress Neuroendocrine Organ Tumors. Mol. Cell. Biol. 2006, 27, 1495–1504. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.; Heller, R.S.; Lechan, R.M.; Heilman, C.B. Regression of a nonfunctioning pituitary macroadenoma on the CDK4/6 inhibitor palbociclib: Case report. Neurosurg. Focus 2018, 44, E9. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.-A.; Jiang, H.; Ben-Shlomo, A.; Wawrowsky, K.; Fan, X.-M.; Lin, S.; Melmed, S. Targeting zebrafish and murine pituitary corticotroph tumors with a cyclin-dependent kinase (CDK) inhibitor. Proc. Natl. Acad. Sci. USA 2011, 108, 8414–8419. [Google Scholar] [CrossRef] [Green Version]
- Lamb, L.S.; Sim, H.-W.; I McCormack, A. Exploring the Role of Novel Medical Therapies for Aggressive Pituitary Tumors: A Review of the Literature—“Are We There Yet?”. Cancers 2020, 12, 308. [Google Scholar] [CrossRef] [Green Version]
- Dineen, R.; Stewart, P.M.; Sherlock, M. Factors impacting on the action of glucocorticoids in patients receiving glucocorticoid therapy. Clin. Endocrinol. 2018, 90, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Asa, S.L.; Ezzat, S. Vitamin D and its analog EB1089 induce p27 accumulation and diminish association of p27 with Skp2 independent of PTEN in pituitary corticotroph cells. Brain Pathol. 2002, 12, 412–419. [Google Scholar] [CrossRef]
- Muşat, M.; Vax, V.V.; Borboli, N.; Gueorguiev, M.; Bonner, S.; Korbonits, M.; Grossman, A.B. Cell cycle dysregulation in pituitary oncogenesis. Front. Horm. Res. 2004, 32, 34–62. [Google Scholar] [CrossRef] [PubMed]
- Prada, E.T.A.; Nölting, S.; Spoettl, G.; Maurer, J.; Auernhammer, C.J. The Novel Cyclin-Dependent Kinase 4/6 Inhibitor Ribociclib (LEE011) Alone and in Dual-Targeting Approaches Demonstrates Antitumoral Efficacy in Neuroendocrine Tumors in vitro. Neuroendocrinology 2017, 106, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.; Nguyen, L.S.; Wassermann, J.; Spano, J.-P.; Funck-Brentano, C.; Salem, J.-E. Cardiac arrhythmia considerations of hormone cancer therapies. Cardiovasc. Res. 2019, 115, 878–894. [Google Scholar] [CrossRef]
- Santoni, M.; Occhipinti, G.; Romagnoli, E.; Miccini, F.; Scoccia, L.; Giulietti, M.; Principato, G.; Saladino, T.; Piva, F.; Battelli, N. Different Cardiotoxicity of Palbociclib and Ribociclib in Breast Cancer: Gene Expression and Pharmacological Data Analyses, Biological Basis, and Therapeutic Implications. BioDrugs 2019, 33, 613–620. [Google Scholar] [CrossRef]
- Raschi, E.; Vasina, V.; Poluzzi, E.; De Ponti, F. The hERG K+ channel: Target and antitarget strategies in drug development. Pharmacol. Res. 2008, 57, 181–195. [Google Scholar] [CrossRef]
- Martinson, A.S.; Van Rossum, D.B.; Diatta, F.H.; Layden, M.J.; Rhodes, S.A.; Martindale, M.Q.; Jegla, T.J. Functional evolution of Erg potassium channel gating reveals an ancient origin for IKr. Proc. Natl. Acad. Sci. USA 2014, 111, 5712–5717. [Google Scholar] [CrossRef] [Green Version]
- Hardman, R.M.; Forsythe, I.D. Ether-à-go-go-related gene K+ channels contribute to threshold excitability of mouse auditory brainstem neurons. J. Physiol. 2009, 587, 2487–2497. [Google Scholar] [CrossRef]
- Bauer, C.K.; Schwarz, J.R. Ether-à-go-goK+channels: Effective modulators of neuronal excitability. J. Physiol. 2018, 596, 769–783. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, C.; Bal, R. ERG Channels Regulate Excitability in Stellate and Bushy Cells of Mice Ventral Cochlear Nucleus. J. Membr. Biol. 2018, 251, 711–722. [Google Scholar] [CrossRef]
- Wu, S.-N.; Jan, C.-R.; Li, H.-F.; Chiang, H.-T. Characterization of Inhibition by Risperidone of the Inwardly Rectifying K+ Current in Pituitary GH3 Cells. Neuropsychopharmacology 2000, 23, 676–689. [Google Scholar] [CrossRef]
- Wu, S.-N.; Yang, W.-H.; Yeh, C.-C.; Huang, H.-C. The inhibition by di(2-ethylhexyl)-phthalate of erg-mediated K+ current in pituitary tumor (GH3) cells. Arch. Toxicol. 2012, 86, 713–723. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-Z.; Jiang, M.; Zhen, Y.-S. HERG K+ channel expression-related chemosensitivity in cancer cells and its modulation by erythromycin. Cancer Chemother. Pharmacol. 2005, 56, 212–220. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Cao, L.; Han, H.; Wang, J.; Yang, B.; Nattel, S.; Wang, Z. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002, 62, 4843–4848. [Google Scholar] [PubMed]
- Garcia-Quiroz, J. Astemizole: An Old Anti-histamine as a New Promising Anti-cancer Drug. Anti Cancer Agents Med. Chem. 2011, 11, 307–314. [Google Scholar] [CrossRef]
- A Hibberts, N.; Simpson, D.J.; E Bicknell, J.; Broome, J.C.; Hoban, P.R.; Clayton, R.; E Farrell, W. Analysis of cyclin D1 (CCND1) allelic imbalance and overexpression in sporadic human pituitary tumors. Clin. Cancer Res. 1999, 5, 2133–2139. [Google Scholar] [PubMed]
- Ramsey, M.R.; Krishnamurthy, J.; Pei, X.-H.; Torrice, C.; Lin, W.; Carrasco, D.R.; Ligon, K.L.; Xiong, Y.; Sharpless, N.E. Expression of p16Ink4a Compensates for p18Ink4c Loss in Cyclin-Dependent Kinase 4/6-Dependent Tumors and Tissues. Cancer Res. 2007, 67, 4732–4741. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, N.; Ravindran, E.; Zaqout, S.; Neubert, G.; Schindler, D.; Ninnemann, O.; Gräf, R.; Seiler, A.E.M.; Kaindl, A.M. Loss of CDK5RAP2 affects neural but not non-neural mESC differentiation into cardiomyocytes. Cell Cycle 2015, 14, 2044–2057. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; Lin, M.-W.; Lin, A.-A.; Peng, H.; Wu, S.-N. Evidence for state-dependent block of DPI 201-106, a synthetic inhibitor of Na+ channel inactivation, on delayed-rectifier K+ current in pituitary tumor (GH3) cells. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59, 409–423. [Google Scholar]
- Yang, C.-S.; Lai, M.-C.; Liu, P.-Y.; Lo, Y.-C.; Huang, C.-W.; Wu, S.-N. Characterization of the Inhibitory Effect of Gastrodigenin and Gastrodin on M-type K+ Currents in Pituitary Cells and Hippocampal Neurons. Int. J. Mol. Sci. 2019, 21, 117. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-T.; Wu, S.-N. Activation of voltage-gated sodium current and inhibition oferg-mediated potassium current caused by telmisartan, an antagonist of angiotensin II type-1 receptor, in HL-1 atrial cardiomyocytes. Clin. Exp. Pharmacol. Physiol. 2018, 45, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Chern, J.-H.; Shen, S.; Chen, H.-H.; Hsu, Y.-T.; Lee, C.-C.; Chen, H.-H.; Lai, M.-C.; Shie, F.-S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell. Physiol. 2017, 232, 3409–3421. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Liu, P.-Y.; Gao, Z.-H.; Lee, S.-W.; Lee, W.-K.; Wu, S.-N. Evidence for the Effectiveness of Remdesivir (GS-5734), a Nucleoside-Analog Antiviral Drug in the Inhibition of IK(M) or IK(DR) and in the Stimulation of IMEP. Front. Pharmacol. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Brown, B.S.; McKinnon, D.; Cohen, I.S. Molecular basis for differential sensitivity of KCNQ and I(Ks) channels to the cognitive enhancer XE991. Mol. Pharmacol. 2000, 57, 1218–1223. [Google Scholar] [PubMed]
- Wu, S.-N.; Li, H.-F.; Jan, C.-R.; Chen, I.-J.; Lo, Y.-C. Selective block by glyceryl nonivamide of inwardly rectifying K+ current in rat anterior pituitary GH3 cells. Life Sci. 1998, 63, PL281–PL288. [Google Scholar] [CrossRef]
- Chen, B.-S.; Lo, Y.-C.; Peng, H.; Hsu, T.-I.; Wu, S.-N. Effects of Ranolazine, a Novel Anti-anginal Drug, on Ion Currents and Membrane Potential in Pituitary Tumor GH3 Cells and NG108-15 Neuronal Cells. J. Pharmacol. Sci. 2009, 110, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.-T.; Lo, Y.-C.; Wu, S.-N. Characterization of Convergent Suppression by UCL-2077 (3-(Triphenylmethylaminomethyl)pyridine), Known to Inhibit Slow Afterhyperpolarization, of erg-Mediated Potassium Currents and Intermediate-Conductance Calcium-Activated Potassium Channels. Int. J. Mol. Sci. 2020, 21, 1441. [Google Scholar] [CrossRef] [Green Version]
- Cartee, L.; Wang, Z.; Decker, R.H.; Chellappan, S.P.; Fusaro, G.; Hirsch, K.G.; Sankala, H.M.; Dent, P.; Grant, S. The cyclin-dependent kinase inhibitor (CDKI) flavopiridol disrupts phorbol 12-myristate 13-acetate-induced differentiation and CDKI expression while enhancing apoptosis in human myeloid leukemia cells. Cancer Res. 2001, 61, 2583–2591. [Google Scholar]
- Park, S.J.; Yang, S.W.; Kim, B.-C. Transforming growth factor-β1 induces cell cycle arrest by activating atypical cyclin-dependent kinase 5 through up-regulation of Smad3-dependent p35 expression in human MCF10A mammary epithelial cells. Biochem. Biophys. Res. Commun. 2016, 472, 502–507. [Google Scholar] [CrossRef]
- McDermott, M.S.; Chumanevich, A.A.; Lim, C.-U.; Liang, J.; Chen, M.; Altilia, S.; Oliver, D.; Rae, J.M.; Shtutman, M.; Kiaris, H.; et al. Inhibition of CDK8 mediator kinase suppresses estrogen dependent transcription and the growth of estrogen receptor positive breast cancer. Oncotarget 2017, 8, 12558–12575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannikko, R.; Pandey, S.; Larsson, H.P.; Elinder, F. Hysteresis in the Voltage Dependence of HCN Channels. J. Gen. Physiol. 2005, 125, 305–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.P.; Thouta, S.; Claydon, T. Modulation of hERG K+ Channel Deactivation by Voltage Sensor Relaxation. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Golomb, D.; Donner, K.; Shacham, L.; Shlosberg, D.; Amitai, Y.; Hansel, D. Mechanisms of Firing Patterns in Fast-Spiking Cortical Interneurons. PLoS Comput. Biol. 2007, 3, e156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-N.; Chen, B.-S.; Lin, M.-W.; Liu, Y. Contribution of slowly inactivating potassium current to delayed firing of action potentials in NG108-15 neuronal cells: Experimental and theoretical studies. J. Theor. Biol. 2008, 252, 711–721. [Google Scholar] [CrossRef]
- Van Ginneken, A.C.; Giles, W. Voltage clamp measurements of the hyperpolarization-activated inward current I(f) in single cells from rabbit sino-atrial node. J. Physiol. 1991, 434, 57–83. [Google Scholar] [CrossRef]
- Lu, T.-L.; Wu, S.-N.; Lu, T.-J. Inhibitory Effective Perturbations of Cilobradine (DK-AH269), A Blocker of HCN Channels, on the Amplitude and Gating of Both Hyperpolarization-Activated Cation and Delayed-Rectifier Potassium Currents. Int. J. Mol. Sci. 2020, 21, 2416. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-T.; Gao, Z.-H.; Lo, Y.-C.; Wu, S.-N. Evidence for Effective Inhibitory Actions on Hyperpolarization-Activated Cation Current Caused by Ganoderma Triterpenoids, the Main Active Constitutents of Ganoderma Spores. Molecules 2019, 24, 4256. [Google Scholar] [CrossRef] [Green Version]
- Lehnhoff, J.; Strauss, U.; Wierschke, S.; Grosser, S.; Pollali, E.; Schneider, U.C.; Holtkamp, M.; Dehnicke, C.; Deisz, R.A. The anticonvulsant lamotrigine enhances Ih in layer 2/3 neocortical pyramidal neurons of patients with pharmacoresistant epilepsy. Neuropharmacology 2019, 144, 58–69. [Google Scholar] [CrossRef]
- Martínez-Chávez, A.; Van Hoppe, S.; Rosing, H.; Lebre, M.C.; Tibben, M.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein Limits Ribociclib Brain Exposure and CYP3A4 Restricts Its Oral Bioavailability. Mol. Pharm. 2019, 16, 3842–3852. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Searle, K.; Jerzak, K.J. Central nervous system-specific efficacy of CDK4/6 inhibitors in randomized controlled trials for metastatic breast cancer. Oncotarget 2019, 10, 6317–6322. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.T.; Davis, A.; Baker, S.J.; Campagne, O.; Stewart, C.F. CNS penetration of the CDK4/6 inhibitor ribociclib in non-tumor bearing mice and mice bearing pediatric brain tumors. Cancer Chemother. Pharmacol. 2019, 84, 447–452. [Google Scholar] [CrossRef]
- Miller, T.W.; Traphagen, N.A.; Li, J.; Lewis, L.D.; Lopes, B.; Asthagiri, A.; Loomba, J.; De Jong, J.; Schiff, D.; Patel, S.H.; et al. Tumor pharmacokinetics and pharmacodynamics of the CDK4/6 inhibitor ribociclib in patients with recurrent glioblastoma. J. Neuro-Oncol. 2019, 144, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Gervaso, L.; Montero, A.J.; Jia, X.; Khorana, A.A. Venous thromboembolism in breast cancer patients receiving cyclin-dependent kinase inhibitors. J. Thromb. Haemost. 2019, 18, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Curigliano, G. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.-T.; Lo, Y.-C.; Gao, Z.-H.; Wu, S.-N. Evidence for the Capability of Roxadustat (FG-4592), an Oral HIF Prolyl-Hydroxylase Inhibitor, to Perturb Membrane Ionic Currents: An Unidentified yet Important Action. Int. J. Mol. Sci. 2019, 20, 6027. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Martínez, C.; Lallena, M.J.; Sanfeliciano, S.G.; De Dios, A. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs: Recent advances (2015–2019). Bioorganic Med. Chem. Lett. 2019, 29, 126637. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Yin, C.; An, B.; Hao, Y.; Wei, T.; Li, L.; Song, G. Arsenic trioxide inhibits breast cancer cell growth via microRNA-328/hERG pathway in MCF-7 cells. Mol. Med. Rep. 2012, 12, 1233–1238. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.-Y.; Chang, W.-T.; Wu, S.-N. Characterization of the Synergistic Inhibition of IK(erg) and IK(DR) by Ribociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor. Int. J. Mol. Sci. 2020, 21, 8078. https://doi.org/10.3390/ijms21218078
Liu P-Y, Chang W-T, Wu S-N. Characterization of the Synergistic Inhibition of IK(erg) and IK(DR) by Ribociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor. International Journal of Molecular Sciences. 2020; 21(21):8078. https://doi.org/10.3390/ijms21218078
Chicago/Turabian StyleLiu, Pin-Yen, Wei-Ting Chang, and Sheng-Nan Wu. 2020. "Characterization of the Synergistic Inhibition of IK(erg) and IK(DR) by Ribociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor" International Journal of Molecular Sciences 21, no. 21: 8078. https://doi.org/10.3390/ijms21218078