Self-Assembly of Supramolecular Architectures by the Effect of Amino Acid Residues of Quaternary Ammonium Pillar[5]arenes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Water-Soluble Pillar[5]arenes Containing Amino Acid Residues
2.2. Synthesis of Monomeric Analogues
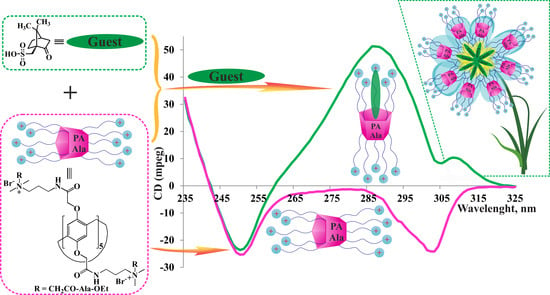
2.3. CD Spectroscopy
2.4. Complexation and Aggregation of Water-Soluble Pillar[5]arenes Studied by UV-, NMR Spectroscopy and Dynamic Light Scattering
2.4.1. UV Spectroscopy
2.4.2. NMR Study of pillar[5]arene Complexes
2.4.3. Aggregation Study of pillar[5]arenes Containing Amino Acid Residues with Guests by DLS and TEM
2.4.4. Theoretical Chemical Calculations
2.4.5. CD Spectroscopy
3. Materials and Methods
3.1. General
3.2. General Procedure for the Synthesis of pillar[5]arenes 12–15
3.3. General Procedure for the Synthesis of Compounds 17 and 18
3.4. UV-Spectroscopy
3.4.1. Determination of the Stability Constant and Stoichiometry of the Complex by Spectrophotometric Titration
3.4.2. Job Plots
3.5. Computational Methods
3.6. Dynamic Light Scattering (DLS)
3.7. Transmission Electron Microscopy (TEM)
3.8. 1H diffusion Ordered Spectroscopy (DOSY)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gumustas, M.; Ozkan, S.A.; Chankvetadze, B. Analytical and preparative scale separation of enantiomers of chiral drugs by chromatography and related methods. Curr. Med. Chem. 2018, 25, 4152–4188. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, N.; Konwer, S.; Ranawat, H.; Chen, N.-T.; Zhuo, G.-Y. Enantiomeric recognition and separation by chiral nanoparticles. Molecules 2019, 24, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, N.M.; Franco, P.; Lindner, W. Separation of enantiomers: Needs, challenges, perspectives. J. Chromatogr. A 2001, 906, 3–33. [Google Scholar] [CrossRef]
- Dai, J.; Xiong, D.; Yuan, T.; Liu, J.; Chen, T.; Shao, Z. Chiral primary amine catalysis for asymmetric Mannich reactions of aldehydes with ketimines: Stereoselectivity and reactivity. Angew. Chem. Int. Ed. 2017, 56, 12697–12701. [Google Scholar] [CrossRef] [PubMed]
- García-Guirado, J.; Svedendahl, M.; Puigdollers, J.; Quidant, R. Enantiomer-selective molecular sensing using racemic nanoplasmonic arrays. Nano Lett. 2018, 18, 6279–6285. [Google Scholar] [CrossRef] [Green Version]
- Octa-Smolin, F.; Thiele, M.; Yadav, R.; Platzek, A.; Clever, G.H.; Niemeyer, J. Chiral receptors for lysine based on covalently linked bis- and tris-binaphthylphosphoric acids. Org. Lett. 2018, 20, 6153–6156. [Google Scholar] [CrossRef]
- Amendola, V.; Boiocchi, M.; Esteban-Gomez, D.; Fabbrizzi, L.; Monzani, E. Chiral receptors for phosphate ions. Org. Biomol. Chem. 2005, 3, 2632–2639. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Yuan, Y.-X.; Wang, W.; Li, D.-M.; Zhang, H.-C.; Wu, B.-X.; Liu, M.; Zheng, Y.-S. Chiral recognition and enantiomer excess determination based on emission wavelength change of AIEgen rotor. Nat. Commun. 2020, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Kutovaya, I.V.; Zakharova, E.A.; Shmatova, O.I.; Nenajdenko, V.G. Macrocyclic pseudopeptides having proline or pipecolic acid residues. Efficient synthesis via Ugi-click strategy. Eur. J. Org. Chem. 2019, 2019, 4855–4862. [Google Scholar] [CrossRef]
- Webster, A.M.; Cobb, S.L. Recent advances in the synthesis of peptoid macrocycles. Chem. Eur. J. 2018, 24, 7560–7573. [Google Scholar] [CrossRef]
- Hayakawa, M.; Ohsawa, A.; Takeda, K.; Torii, R.; Kitamura, Y.; Katagiri, H.; Ikeda, M. Cyclic arylopeptoid oligomers: Synthesis and conformational propensities of peptide-mimetic aromatic macrocycles. Org. Biomol. Chem. 2018, 16, 8505–8512. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Jie, K.; Zhou, Y.; Zhou, J.; Xia, D.; Huang, F. Nanoparticles with near-infrared emission enhanced by pillararene-based molecular recognition in water. J. Am. Chem. Soc. 2016, 138, 80–83. [Google Scholar] [CrossRef]
- Gunasekara, R.W.; Zhao, Y. A General method for selective recognition of monosaccharides and oligosaccharides in water. J. Am. Chem. Soc. 2017, 139, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, L.; Huo, M.; Zeng, M.; Peng, L.; Feng, A.; Wang, X.; Yuan, J. Direct synthesis of polymer nanotubes by aqueous dispersion polymerization of a cyclodextrin/styrene complex. Angew. Chem. Int. Ed. 2017, 56, 16541–16545. [Google Scholar] [CrossRef] [PubMed]
- Assaf, K.I.; Alnajjar, M.A.; Nau, W.M. Supramolecular assemblies through host–guest complexation between cucurbiturils and an amphiphilic guest molecule. Chem. Commun. 2018, 54, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Durso, A.; Brancatelli, G.; Hickey, N.; Farnetti, E.; Zorzi, R.D.; Bonaccorso, C.; Purrello, R.; Geremia, S. Interactions of a water-soluble calix[4]arene with spermine: Solution and solid-state characterization. Supramol. Chem. 2016, 28, 499–505. [Google Scholar] [CrossRef]
- Mostovaya, O.A.; Padnya, P.L.; Shurpik, D.N.; Vavilova, A.A.; Evtugyn, V.G.; Osin, Y.N.; Stoikov, I.I. Iminodiacetic derivatives of p-tert-butylthiacalix[4]arene: Synthesis and influence of conformation on the aggregation with bismarck brown Y. Macroheterocycles 2017, 10, 154–163. [Google Scholar] [CrossRef]
- Burilov, V.A.; Mironova, D.A.; Ibragimova, R.R.; Evtugyn, V.G.; Osin, Y.N.; Solovieva, S.E.; Antipin, I.S. Imidazolium p-tert-butylthiacalix[4]arene amphiphiles-aggregation in water solutions and binding with adenosine 5′-triphosphate dipotassium salt. BioNanoScience 2018, 8, 337–343. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Oguz, M.; Yilmaz, M. New water soluble p-sulphonatocalix[4]arene chemosensor appended with rhodamine for selective detection of Hg2+ ion. J. Mol. Struct. 2020, 1203, 127436. [Google Scholar] [CrossRef]
- Nazarova, A.A.; Yakimova, L.S.; Padnya, P.L.; Evtugyn, V.G.; Osin, Y.N.; Cragg, P.J.; Stoikov, I.I. Monosubstituted pillar[5]arene functionalized with (amino)phosphonate fragments are “smart” building blocks for constructing nanosized structures with some s-and p-metal cations in the organic phase. New J. Chem. 2019, 43, 14450–14458. [Google Scholar] [CrossRef]
- Jin, X.-Y.; Song, N.; Wang, X.; Wang, C.-Y.; Wang, Y.; Yang, Y.-W. Monosulfonicpillar[5]arene: Synthesis, characterization, and complexation with tetraphenylethene for aggregation-induced emission. Sci. Rep. 2018, 8, 4035. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Q.; Gao, Z.; Wang, H.; Shi, B.; Wu, Y.; Shangguan, L.; Hong, X.; Wang, F.; Huang, F. Pillararene host–guest complexation induced chirality amplification: A new way to detect cryptochiral compounds. Angew. Chem. Int. Ed. 2020, 59, 10868–10872. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Czaplewska, A.; Wang, L.; Schubert, S.; Brendel, J.C.; Schubert, U.S. Straightforward access to glycosylated, acid sensitive nanogels by host–guest interactions with sugar-modified pillar[5]arenes. ACS Macro Lett. 2020, 9, 540–545. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Lee, S.S.; Jung, J.H. Critical role of achiral guest molecules in planar chirality inversion of alanine-appended pillar[5]arenes. Org. Lett. 2019, 21, 1232–1236. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Lv, S.; Zeng, X.; Sun, Y.; Li, H.; Zhang, R. The chiral interfaces fabricated by D/L-alanine pillar[5]arenes for selectivity adsorbing ctDNA. Chem. Commun. 2019, 55, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, F.; Quan, J.; Zhu, F.; Hong, W.; Ma, J.; Pang, H.; Sun, Y.; Tian, D.; Li, H. A biomimetic chiral-driven ionic gate constructed by pillar[6]arene-based host-guest system. Nat. Commun. 2018, 9, 2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-Y.; Haoyang, W.-W.; Zhang, M.; Wu, G.; Li, Z.-T.; Hou, J.-L. Synthetic channel that efficiently inserts into mammalian cell membranes and destroys cancer cells. Faradey Discuss. 2018, 209, 149–159. [Google Scholar] [CrossRef]
- Si, W.; Li, Z.-T.; Hou, J.-L. Voltage-driven reversible insertion into and leaving from a lipid bilayer: Tuning transmembrane transport of artificial channels. Angew. Chem. Int. Ed. 2014, 53, 4578–4581. [Google Scholar] [CrossRef]
- Yang, K.; Chang, Y.; Wen, J.; Lu, Y.; Pei, Y.; Cao, S.; Wang, F.; Pei, Z. Supramolecular vesicles based on complex of Trp-modified pillar[5]arene and galactose derivative for synergistic and targeted drug delivery. Chem. Mater. 2016, 28, 1990–1993. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Padnya, P.L.; Basimova, L.T.; Evtugin, V.G.; Plemenkov, V.V.; Stoikov, I.I. Synthesis of new decasubstituted pillar[5]arenes containing glycine fragments and their interaction with Bismarck brown Y. Mendeleev Commun. 2015, 25, 432–434. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Sevastyanov, D.A.; Zelenikhin, P.V.; Subakaeva, E.V.; Evtugyn, V.G.; Osin, Y.N.; Gragg, P.J.; Stoikov, I.I. Hydrazides of glycine-containing decasubstituted pillar[5]arenes: Synthesis and encapsulation of Floxuridine. Tetrahedron Lett. 2018, 59, 4410–4415. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Shurpik, D.N.; Makhmutova, A.R.; Stoikov, I.I. pillar[5]arenes bearing amide and carboxylic groups as synthetic receptors for alkali metal ions. Macroheterocycles 2017, 10, 226–232. [Google Scholar] [CrossRef]
- Ogoshi, T. para-Bridged symmetrical pillar[5]arenes: Their Lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Nazarova, A.A.; Padnya, P.L.; Gilyazeva, A.I.; Khannanov, A.A.; Evtugyn, V.G.; Kutyreva, M.P.; Klochkov, V.V.; Stoikov, I.I. Supramolecular motifs for the self-assembly of monosubstituted pillar[5]arenes with an amide fragment: From nanoparticles to supramolecular polymers. New J. Chem. 2018, 42, 19853–19863. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Nazarova, A.A.; Makhmutova, L.I.; Kiznyaev, V.N.; Stoikov, I.I. Uncharged water-soluble amide derivatives of pillar[5]arene: Synthesis and supramolecular self-assembly with tetrazole-containing polymers. Russ. Chem. Bull. 2019, 69, 97–104. [Google Scholar] [CrossRef]
- Padnya, P.L.; Bayarashov, E.E.; Zueva, I.V.; Lushchekina, S.V.; Lenina, O.A.; Evtugyn, V.G.; Osin, Y.N.; Petrov, K.A.; Stoikov, I.I. Water-soluble betaines and amines based on thiacalix[4]arene scaffold as new cholinesterase inhibitors. Bioorg. Chem. 2020, 94, 103455. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Shi, B.; Shangguan, L.; Tong, W.; Yu, G.; Mao, Z.; Huang, F. Supramolecular peptide constructed by molecular Lego allowing programmable self-assembly for photodynamic therapy. Nat. Commun. 2019, 10, 2412. [Google Scholar] [CrossRef] [Green Version]
- Kaverzneva, E.D.; Zvorykina, V.K.; Kiseleva, V.V. Synthesis of N-(6-benzylthio-9H-9-purinyl) acetylamino acids. Bull. Acad. Sci. USSR Div. Chem. Sci. 1970, 10, 2157–2166. [Google Scholar] [CrossRef]
- Li, C.; Shu, X.; Li, J.; Chen, S.; Han, K.; Xu, M.; Jia, X. Complexation of 1,4-Bis(pyridinium)butanes by negatively charged carboxylatopillar[5]arene. J. Org. Chem. 2011, 76, 8458–8465. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Yakimova, L.S.; Rizvanov, I.K.; Plemenkov, V.V.; Stoiko, I.I. Water-soluble pillar[5]arenes: Synthesis and characterization of the inclusion complexes with p-toluenesulfonic acid. Macroheterocycles 2015, 8, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Shurpik, D.N.; Padnya, P.L.; Evtugyn, V.G.; Mukhametzyanov, T.A.; Khannanov, A.A.; Kutyreva, M.P.; Stoikov, I.I. Synthesis and properties of chiral nanoparticles based on (pS)- and (pR)-decasubstituted pillar[5]arenes containing secondary amide fragments. RSC Adv. 2016, 6, 9124–9131. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Shurpik, D.N.; Gilmanova, L.H.; Makhmutova, A.R.; Rakhimbekova, A.; Stoikov, I.I. Highly selective binding of methyl orange dye by cationic water-soluble pillar[5]arenes. Org. Biomol. Chem. 2016, 14, 4233–4238. [Google Scholar] [CrossRef] [Green Version]
- Gómez-González, B.; Francisco, V.; Montecinos, R.; García-Río, L. Investigation of the binding modes of a positively charged pillar[5]arene: Internal and external guest complexation. Org. Biomol. Chem. 2017, 15, 911–919. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, F.; Yang, F.; Ma, D. Carboxylated pillar[n]arene (n = 5–7) host molecules: High affinity and selective binding in water. Org. Biomol. Chem. 2019, 17, 5106–5111. [Google Scholar] [CrossRef]
- Bindfit v0.5. Available online: http://supramolecular.org/bindfit (accessed on 27 November 2019).
- Dais, P.; Misiak, M.; Hatzakis, E. Analysis of marine dietary supplements using NMR spectroscopy. Anal. Methods. 2015, 7, 5226–5238. [Google Scholar] [CrossRef]
- Diasa, R.S.; Pais, C.C.; Miguela, M.G.; Lindman, B. DNA and surfactants in bulk and at interfaces. Colloids Surf. A Physicochem. Eng. Asp. 2004, 250, 115–131. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.; Renggli, K.; Razumovitch, J.; Vebert, C. Dynamic Light Scattering in Supramolecular Materials Chemistry. Supramolecular Chemistry: From Molecules to Nanomaterials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 411–424. [Google Scholar]
- Schönbeck, C.; Li, H.; Han, B.-H.; Laursen, B.W. Solvent Effects and Driving Forces in Pillararene Inclusion Complexes. J. Phys. Chem. B 2015, 119, 6711–6720. [Google Scholar] [CrossRef]
- Vavayannis, A.; Parissi-Poulou, M.; Papadopoulou-Daifoti, Z.; Noras, I. Synthesis and pharmacological study of new dimethylcarbamates. Eur. J. Med. Chem. 1985, 20, 37–42. [Google Scholar]
- Spartan’18; Wavefunction, Inc.: Irvine, CA, USA, 2018.












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarova, A.; Shurpik, D.; Padnya, P.; Mukhametzyanov, T.; Cragg, P.; Stoikov, I. Self-Assembly of Supramolecular Architectures by the Effect of Amino Acid Residues of Quaternary Ammonium Pillar[5]arenes. Int. J. Mol. Sci. 2020, 21, 7206. https://doi.org/10.3390/ijms21197206
Nazarova A, Shurpik D, Padnya P, Mukhametzyanov T, Cragg P, Stoikov I. Self-Assembly of Supramolecular Architectures by the Effect of Amino Acid Residues of Quaternary Ammonium Pillar[5]arenes. International Journal of Molecular Sciences. 2020; 21(19):7206. https://doi.org/10.3390/ijms21197206
Chicago/Turabian StyleNazarova, Anastasia, Dmitriy Shurpik, Pavel Padnya, Timur Mukhametzyanov, Peter Cragg, and Ivan Stoikov. 2020. "Self-Assembly of Supramolecular Architectures by the Effect of Amino Acid Residues of Quaternary Ammonium Pillar[5]arenes" International Journal of Molecular Sciences 21, no. 19: 7206. https://doi.org/10.3390/ijms21197206