Novel Medicinal Mushroom Blend as a Promising Supplement in Integrative Oncology: A Multi-Tiered Study using 4T1 Triple-Negative Mouse Breast Cancer Model
Abstract
:1. Introduction
2. Results
2.1. Quality of Life
2.2. Histopathology (H&E Staining) and Ultrastructural Analysis (TEM)
2.3. Picrosirius Red Staining: Fibrillar Collagen Networks Evaluation
2.4. Inflammatory Pathway: IL-6 and TGF-β1 Immunohistochemical Assessment
2.5. Oxidative Stress Pathway: SOD1, NOS2, and COX2 Immunohistochemical Assessment
3. Discussion
4. Materials and Methods
4.1. Blend Supplementation
4.2. Cell Culture
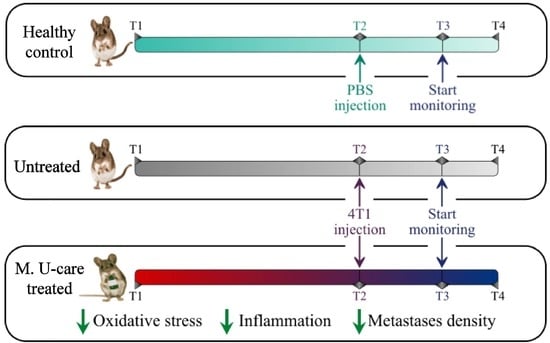
4.3. Animals and Experimental Design
4.4. Emergence Test
4.5. The Quality of Life Index
4.6. Tissue Sampling, Histology, Immunohistochemistry, and Ultrastructural Morphology Evaluations
4.6.1. Lung Specimens Preparation
4.6.2. H&E: Histopathological Observations
4.6.3. Picrosirius Red Staining
4.6.4. Immunohistochemistry: Inflammatory and Oxidative Stress Pathways Assessment
4.6.5. Transmission Electron Microscopy (TEM): UA and LC Staining
4.6.6. Semiquantitative Lung Lesion Analysis
4.6.7. Histochemical and Immunohistochemical Evaluations
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO: Breast cancer. Available online: http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ (accessed on 15 November 2019).
- Cardoso, F.; Harbeck, N.; Fallowfield, L.; Kyriakides, S.; Senkus, E.; ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. 7), vii11–vii19. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Duffy, S.W.; Tabár, L. Breast cancer screening: The evolving evidence. Oncology 2012, 26, 471–475. [Google Scholar] [PubMed]
- Yadav, B.S.; Chanana, P.; Jhamb, S. Biomarkers in triple negative breast cancer: A review. World J. Clin. Oncol. 2015, 6, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Sun, H.; Deng, C.X. Potential therapeutic targets of triple-negative breast cancer based on its intrinsic subtype. Oncotarget 2017, 8, 73329–73344. [Google Scholar] [CrossRef]
- Yao, Y.; Chu, Y.; Xu, B.; Hu, Q.; Song, Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci. Rep. 2019, 39, BSR20190288. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal Mushrooms in Human Clinical Studies. Part I. Anticancer, Oncoimmunological, and Immunomodulatory Activities: A Review. Int. J. Med. Mushrooms 2017, 19, 279–317. [Google Scholar] [CrossRef]
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.L.; Berretta, M. B-glucans from Grifola frondosa and Ganoderma lucidum in breast cancer: An example of complementary and integrative medicine. Oncotarget 2018, 9, 24837–24856. [Google Scholar] [CrossRef] [Green Version]
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget 2018, 9, 29259–29274. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Sliva, D. Novel medicinal mushroom blend suppresses growth and invasiveness of human breast cancer cells. Int. J. Oncol. 2010, 37, 1529–1536. [Google Scholar]
- Alonso, E.N.; Ferronato, M.J.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Guevara, J.A.; Facchinetti, M.M.; Curino, A.C. Antitumoural and antimetastatic activity of Maitake D-Fraction in triple-negative breast cancer cells. Oncotarget 2018, 9, 23396–23412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Li, J.; Gu, B.; Xiao, Y.; Chen, R.; Liu, X.; Xie, X.; Cao, L. Extracts of Cordyceps sinensis inhibit breast cancer cell metastasis via down-regulation of metastasis-related cytokines expression. J. Ethnopharmacol. 2018, 214, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.K. Kawariharatake, Agaricus blazeiMurrill medicinal and dietary effects. Food Rev. Int. 1995, 11, 167–172. [Google Scholar] [CrossRef]
- Firenzuoli, F.; Gori, L.; Lombardo, G. The Medicinal Mushroom Agaricus blazei Murrill: Review of Literature and Pharmaco-Toxicological Problems. Evid. Based Complement. Altern. Med. 2008, 5, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva de Souza, A.C.; Correa, V.G.; Goncalves, G.d.A.; Soares, A.A.; Bracht, A.; Peralta, R.M. Agaricus blazei Bioactive Compounds and their Effects on Human Health: Benefits and Controversies. Curr. Pharm. Des. 2017, 23, 2807–2834. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, B.H.; Kim, G.S.; Jang, J.S.; Kim, S.Y.; Choi, B.D.; Kim, J.O.; Ha, Y.L. Anti-carcinogenic actions of glycoprotein conjugated with isoflavones from submerged-liquid culture of Agaricus blazei mycelia through reciprocal expression of Bcl-2 and Bax proteins. J. Biomed. Res. 2014, 15, 200–206. [Google Scholar] [CrossRef]
- De Sá-Nakanishi, A.B.; Soares, A.A.; de Oliveira, A.L.; Comar, J.F.; Peralta, R.M.; Bracht, A. Effects of treating old rats with an aqueous Agaricus blazei extract on oxidative and functional parameters of the brain tissue and brain mitochondria. Oxid. Med. Cell Longev. 2014, 2014, 563179. [Google Scholar] [CrossRef] [Green Version]
- Ohno, S.; Sumiyoshi, Y.; Hashine, K.; Shirato, A.; Kyo, S.; Inoue, M. Phase I Clinical Study of the Dietary Supplement, Agaricus blazei Murill, in Cancer Patients in Remission. Evid. Based Complement. Altern. Med. 2011, 2011, 192381. [Google Scholar] [CrossRef] [Green Version]
- Ahn, W.S.; Kim, D.J.; Chae, G.T.; Lee, J.M.; Bae, S.M.; Sin, J.I.; Kim, Y.W.; Namkoong, S.E.; Lee, I.P. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int. J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef]
- Jordan, J.L.; Nowak, A.; Lee, T.D.G. Activation of innate immunity to reduce lung metastases in breast cancer. Cancer Immunol. Immunother. 2010, 59, 789–797. [Google Scholar] [CrossRef]
- Wang, J.; Nie, S.; Cui, S.W.; Wang, Z.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. Structural characterization and immunostimulatory activity of a glucan from natural Cordyceps sinensis. Food Hydrocol. 2017, 67, 139–147. [Google Scholar] [CrossRef]
- Li, S.P.; Li, P.; Dong, T.T.; Tsim, K.W. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia. Phytomedicine 2001, 8, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.S.; Shi, L.S.; Kuo, S.C. Cytotoxicity of Ganoderma lucidum triterpenes. J. Nat. Prod. 2001, 64, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Slivova, V.; Harvey, K.; Valachovicova, T.; Sliva, D. Ganoderma lucidum suppresses growth of breast cancer cells through the inhibition of Akt/NF-kappaB signaling. Nutr. Cancer 2004, 49, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Sliva, D.; Sedlak, M.; Slivova, V.; Valachovicova, T.; Lloyd, F.P.; Ho, N.W.Y. Biologic activity of spores and dried powder from Ganoderma lucidum for the inhibition of highly invasive human breast and prostate cancer cells. J. Altern. Complement. Med. 2003, 9, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Q.; Zhao, L.; Huang, X.; Wang, J.; Kang, X. Spore Powder of Ganoderma lucidum Improves Cancer-Related Fatigue in Breast Cancer Patients Undergoing Endocrine Therapy: A Pilot Clinical Trial. Evid. Based Complement. Altern. Med. 2012, 2012, 809614. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Ruiz Beguerie, J.; Sze, D.M.Y.; Chan, G.C.F. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst. Rev. 2016, 4, CD007731. [Google Scholar] [CrossRef]
- Soares, R.; Meireles, M.; Rocha, A.; Pirraco, A.; Obiol, D.; Alonso, E.N.; Joos, G.; Balogh, G. Maitake (D fraction) mushroom extract induces apoptosis in breast cancer cells by BAK-1 gene activation. J. Med. Food 2011, 14, 563–572. [Google Scholar] [CrossRef]
- Alonso, E.N.; Orozco, M.; Nieto, A.E.; Balogh, G.A. Genes Related to Suppression of Malignant Phenotype Induced by Maitake D-Fraction in Breast Cancer Cells. J. Med. Food 2013, 16, 602–617. [Google Scholar] [CrossRef] [Green Version]
- Said, T.K.; Moraes, R.C.; Singh, U.; Kittrell, F.S.; Medina, D. Cyclin-dependent kinase (cdk) inhibitors/cdk4/cdk2 complexes in early stages of mouse mammary preneoplasia. Cell Growth Differ. 2001, 12, 285–295. [Google Scholar]
- Van ’t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.M.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doughtery, E.R.; Jianping, H.; Bittner, M.L. Validation of Computational Methods in Genomics. Curr. Genom. 2007, 8, 1–19. [Google Scholar]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, S.; Cunningham-Rundles, S.; Vickers, A.J.; Cassileth, B. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: Immunological effects. J. Cancer Res. Clin. Oncol. 2009, 135, 1215–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, N.; Li, Q.; Yu, S.; Zhang, J.; He, L.; Ronis, M.J.J.; Badger, T.M. Inhibition of growth and induction of apoptosis in human cancer cell lines by an ethyl acetate fraction from shiitake mushrooms. J. Altern. Complement. Med. 2006, 12, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Li, J.; Song, X.; Zhang, J.; Wang, W.; Wang, X.; Gao, Z.; Jing, H.; Li, S.; Jia, L. The regulation of inflammation and oxidative status against lung injury of residue polysaccharides by Lentinula edodes. Int. J. Biol. Macromol. 2018, 106, 185–192. [Google Scholar] [CrossRef]
- Suzuki, N.; Takimoto, Y.; Suzuki, R.; Arai, T.; Uebaba, K.; Nakai, M.; Strong, J.M.; Tokuda, H. Efficacy of oral administration of Lentinula eododes mycelia extract for breast cancer patients undergoing postoperative hormone therapy. Asian Pac. J. Cancer Prev. 2013, 14, 3469–3472. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, Y.; Maeda, N.; Yamamoto, S.; Yoshino, S.; Oka, M. Evaluation of host quality of life and immune function in breast cancer patients treated with combination of adjuvant chemotherapy and oral administration of Lentinula edodes mycelia extract. Onco Targets Ther. 2013, 6, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, Y.; Yoshino, S.; Yamamoto, S.; Maeda, N.; Azumi, T.; Komoike, Y.; Okuno, K.; Iwasa, T.; Tsurutani, J.; Nakagawa, K.; et al. Lentinula edodes mycelia extract plus adjuvant chmotherapy for breast cancer patients: Results of a randomized study on host quality of life and immune function improvement. Mol. Clin. Oncol. 2017, 7, 359–366. [Google Scholar] [CrossRef]
- Segnani, C.; Ippolito, C.; Antonioli, L.; Pellegrini, C.; Blandizzi, C.; Dolfi, A.; Bernardini, N. Histochemical Detection of Collagen Fibers by Sirius Red/Fast Green Is More Sensitive than van Gieson or Sirius Red Alone in Normal and Inflamed Rat Colon. PLoS ONE 2015, 10, e0144630. [Google Scholar] [CrossRef]
- Pienta, K.J.; McGregor, N.; Axelrod, R.; Axelrod, D.E. Ecological therapy for cancer: Defining tumours using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl. Oncol. 2008, 1, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Zechner, C.; Hernandez, G.; Cánovas, J.; Xie, Y.; Sondhi, V.; Wagner, M.; Stadlbauer, V.; Horvath, A.; Leber, B.; et al. The Hormone FGF21 Stimulates Water Drinking in Response to Ketogenic Diet and Alcohol. Cell Metab. 2018, 27, 1338–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Lee, M.S. FGF21 as a stress hormone: The roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab. J. 2014, 38, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Garza, U.; Torres-Oteros, D.; Yarritu-Gallego, A.; Marrero, P.F.; Haro, D.; Relat, J. Fibroblast Growth Factor 21 and the Adaptive Response to Nutritional Challenges. Int. J. Mol. Sci. 2019, 20, E4692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb–prot4986. [Google Scholar] [CrossRef]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef]
- Krakhmal, N.V.; Zavyalova, M.V.; Denisov, E.V.; Vtorushin, S.V.; Perelmuter, V.M. Cancer Invasion: Patterns and Mechanisms. Acta Nat. 2015, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The two faces of IL-6 in the tumour microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Liubomirski, Y.; Lerrer, S.; Meshel, T.; Rubinstein-Achiasaf, L.; Morein, D.; Wiemann, S.; Körner, C.; Ben-Baruch, A. Tumour-Stroma-Inflammation Networks Promote Pro-metastatic Chemokines and Aggressiveness Characteristics in Triple-Negative Breast Cancer. Front. Immunol. 2019, 10, 757. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Tuñón, I.; Ricote, M.; Ruiz, A.; Fraile, B.; Paniagua, R.; Royuela, M. IL-6, its receptors and its relationship with bcl-2 and bax proteins in infiltrating and in situ human breast carcinoma. Histopathology 2005, 47, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumour-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, A.; Quagliariello, V.; Del Vecchio, V.; Falco, M.; Luciano, A.; Amruthraj, N.J.; Nasti, G.; Ottaiano, A.; Berretta, M.; Iaffaioli, R.V.; et al. Anticancer and Anti-Inflammatory Properties of Ganoderma lucidum Extract Effects on Melanoma and Triple-Negative Breast Cancer Treatment. Nutrients 2017, 9, E210. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.; Cao, D. TGF-beta signaling, tumour microenvironment and tumour progression: The butterfly effect. Front. Biosci. 2010, 15, 180–194. [Google Scholar] [CrossRef] [Green Version]
- Papageorgis, P.; Stylianopoulos, T. Role of TGFβ in regulation of the tumour microenvironment and drug delivery (review). Int. J. Oncol. 2015, 46, 933–943. [Google Scholar] [CrossRef] [Green Version]
- Moore-Smith, L.D.; Isayeva, T.; Lee, J.H.; Frost, A.; Ponnazhagan, S. Silencing of TGF-β1 in tumour cells impacts MMP-9 in tumour microenvironment. Sci. Rep. 2017, 7, 8678. [Google Scholar] [CrossRef] [Green Version]
- Massagué, J.; Gomis, R.R. The logic of TGFbeta signaling. FEBS Lett. 2006, 580, 2811–2820. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Huang, T.; Shen, Y.; Liu, Y.; Zhou, F.; Jin, Y.; Sattar, H.; Wei, Y. Reactive Oxygen Species-Mediated Tumour Microenvironment Transformation: The Mechanism of Radioresistant Gastric Cancer. Oxid. Med. Cell Longev. 2018, 2018, 5801209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kepinska, M.; Kizek, R.; Milnerowicz, H. Metallothionein and Superoxide Dismutase-Antioxidative Protein Status in Fullerene-Doxorubicin Delivery to MCF-7 Human Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, E3253. [Google Scholar] [CrossRef] [Green Version]
- Basudhar, D.; Bharadwaj, G.; Somasundaram, V.; Cheng, R.Y.S.; Ridnour, L.A.; Fujita, M.; Lockett, S.J.; Anderson, S.K.; McVicar, D.W.; Wink, D.A. Understanding the tumour micro-environment communication network from an NOS2/COX2 perspective. Br. J. Pharm. 2019, 176, 155–176. [Google Scholar] [CrossRef]
- Esbona, K.; Yi, Y.; Saha, S.; Yu, M.; Van Doorn, R.R.; Conklin, M.W.; Graham, D.S.; Wisinski, K.B.; Ponik, S.M.; Eliceiri, K.W.; et al. The Presence of Cyclooxygenase 2, Tumour-Associated Macrophages, and Collagen Alignment as Prognostic Markers for Invasive Breast Carcinoma Patients. Am. J. Pathol. 2018, 188, 559–573. [Google Scholar] [CrossRef] [Green Version]
- Brüne, B.; Courtial, N.; Dehne, N.; Syed, S.N.; Weigert, A. Macrophage NOS2 in Tumour Leukocytes. Antioxid. Redox Signal. 2017, 26, 1023–1043. [Google Scholar] [CrossRef]
- Thomas, D.D.; Wink, D.A. NOS2 as an Emergent Player in Progression of Cancer. Antioxid. Redox Signal. 2017, 26, 963–965. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Yeh, H.W.; Lee, S.S.; Chang, C.Y.; Lang, Y.D.; Jou, Y.S. A New Switch for TGFβ in Cancer. Cancer Res. 2019, 79, 3797–3805. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Chen, H.; Lin, J.; Xu, H.; Wu, H.; Bao, G.; Li, J.; Deng, X.; Shui, X.; Gao, W.; et al. FGF21 augments autophagy in random-pattern skin flaps via AMPK signalling pathways and improves tissue survival. Cell Death Dis. 2019, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.J.; Choi, M.R.; Park, H.; Kim, M.; Hong, J.E.; Lee, J.Y.; Chun, H.S.; Lee, K.W.; Yoon Park, J.H. Dietary fat increases solid tumor growth and metastasis of 4T1 murine mammary carcinoma cells and mortality in obesity-resistant BALB/c mice. Breast Cancer Res. 2011, 13, R78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigjeh, S.E.; Yeap, S.K.; Nordin, N.; Rahman, H.; Rosli, R. In Vivo Anti-Tumor Effects of Citral on 4T1 Breast Cancer Cells via Induction of Apoptosis and Downregulation of Aldehyde Dehydrogenase Activity. Molecules 2019, 24, E3241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Guo, F.; Chen, H.; Lin, Y.; Fu, X.; Zhang, H.; Ding, M. Core needle biopsy promotes lung metastasis of breast cancer: An experimental study. Mol. Clin. Oncol. 2019, 10, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roda, E.; Bottone, M.G.; Biggiogera, M.; Milanesi, G.; Coccini, T. Pulmonary and hepatic effects after low dose exposure to nanosilver: Early and long-lasting histological and ultrastructural alterations in rat. Toxicol. Rep. 2019, 6, 1047–1060. [Google Scholar] [CrossRef]
- Brandalise, F.; Cesaroni, V.; Gregori, A.; Repetti, M.; Romano, C.; Orrù, G.; Botta, L.; Girometta, C.; Guglielminetti, M.L.; Savino, E.; et al. Dietary Supplementation of Hericium erinaceus Increases Mossy Fiber-CA3 Hippocampal Neurotransmission and Recognition Memory in Wild-Type Mice. Evid. Based Complement. Altern. Med. 2017, 2017, 3864340. [Google Scholar] [CrossRef] [Green Version]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium erinaceus Improves Recognition Memory and Induces Hippocampal and Cerebellar Neurogenesis in Frail Mice during Aging. Nutrients 2019, 11, E715. [Google Scholar] [CrossRef] [Green Version]
- Weibel, E.R. Morphometry of the human lung: The state of the art after two decades. Bull. Eur. Physiopathol. Respir. 1979, 15, 999–1013. [Google Scholar]
- Avwioro, G. Histochemical Uses of Haematoxylin-A Review. JPCS 2011, 1, 24–34. [Google Scholar]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Healthy Control | Untreated | M. U-care Treated | p Value | |
|---|---|---|---|---|
| Alveolar structure alteration | − | +++ | ++ | * |
| Haemorrhagic foci | − | ++ | ++ | ns |
| Bronchiolar desquamation | − | ++ | + | * |
| Number of pulmonary metastases | − | ++ | + | * |
| Fungal Species | ID Code | Weight % Used in Blend | Part Used |
|---|---|---|---|
| Agaricus blazei | 7700 | 20% | Sporophores |
| Cordyceps sinensis | Cm2 | 20% | Sporophores and mycelia |
| Ganoderma lucidum | Gač | 20% | Sporophores |
| Grifola frondosa | Gf3 | 20% | Sporophores |
| Lentinula edodes | Le.ed.1 | 20% | Sporophores |
| Components | Per Capsule (mg) |
|---|---|
| Ganoderma lucidum | 150 mg |
| Grifola frondosa | 150 mg |
| Agaricus blazei | 150 mg |
| Cordyceps sinensis | 150 mg |
| Lentinula edodes | 150 mg |
| Titled in polysaccharides (α plus β) | >30% |
| 1,3-1,6 Beta-glucans | >15% |
| Antigen | Immunogen | Manufacturer, Species, Mono-polyclonal, cat./lot. No., RRID | Diluted Used | |
|---|---|---|---|---|
| Primary antibodies | Anti-Interleukin-6 (M-19) | Purified antibody raised against a peptide mapping at the C-terminus of IL-6 of mouse origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Goat polyclonal IgG, Cat# sc-1265, RRID: AB_2127470 | 1:100 |
| Anti-Transforming Growth Factor β1 (V) | Purified antibody raised against a peptide mapping at the C-terminus of TGF-β1 of human origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Rabbit polyclonal IgG, Cat# sc-146, RRID: AB_632486 | 1:100 | |
| Anti-Superoxide Dismutase-1 (FL-154) | Purified antibody raised against amino acids 1–154 representing full length SOD1 of human origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Rabbit polyclonal IgG, Cat# sc-11407, RRID: AB_2193779 | 1:100 | |
| Anti-Nitric Oxide Synthases-2 (M19) | Purified antibody raised against a peptide mapping at the C-terminus of NOS2 of mouse origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Rabbit polyclonal IgG, Cat# sc-650, RRID: AB_631831 | 1:100 | |
| Anti-Cyclooxygenase-2(M-19) | Purified antibody raised against a peptide mapping at the C-terminus of COX2 of mouse origin | Santa Cruz Biotechnology (Santa Cruz, CA, USA), Goat polyclonal IgG, Cat# sc-1747, RRID: AB_2084976 | 1:100 | |
| Secondary antibodies | Biotinylated goat anti-rabbit IgG | Gamma immunoglobulin | Vector Laboratories (Burlingame, CA, USA), Goat, lot# PK-6101, RRID: AB_2336820 | 1:200 |
| Biotinylated rabbit anti-goat IgG | Gamma immunoglobulin | Vector Laboratories (Burlingame, CA, USA), Rabbit, Cat# PK-6105, RRID: AB_2336824 | 1:200 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roda, E.; De Luca, F.; Di Iorio, C.; Ratto, D.; Siciliani, S.; Ferrari, B.; Cobelli, F.; Borsci, G.; Priori, E.C.; Chinosi, S.; et al. Novel Medicinal Mushroom Blend as a Promising Supplement in Integrative Oncology: A Multi-Tiered Study using 4T1 Triple-Negative Mouse Breast Cancer Model. Int. J. Mol. Sci. 2020, 21, 3479. https://doi.org/10.3390/ijms21103479
Roda E, De Luca F, Di Iorio C, Ratto D, Siciliani S, Ferrari B, Cobelli F, Borsci G, Priori EC, Chinosi S, et al. Novel Medicinal Mushroom Blend as a Promising Supplement in Integrative Oncology: A Multi-Tiered Study using 4T1 Triple-Negative Mouse Breast Cancer Model. International Journal of Molecular Sciences. 2020; 21(10):3479. https://doi.org/10.3390/ijms21103479
Chicago/Turabian StyleRoda, Elisa, Fabrizio De Luca, Carmine Di Iorio, Daniela Ratto, Stella Siciliani, Beatrice Ferrari, Filippo Cobelli, Giuseppina Borsci, Erica Cecilia Priori, Silvia Chinosi, and et al. 2020. "Novel Medicinal Mushroom Blend as a Promising Supplement in Integrative Oncology: A Multi-Tiered Study using 4T1 Triple-Negative Mouse Breast Cancer Model" International Journal of Molecular Sciences 21, no. 10: 3479. https://doi.org/10.3390/ijms21103479