Protective Effect of Tetrahydroquinolines from the Edible Insect Allomyrina dichotoma on LPS-Induced Vascular Inflammatory Responses
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Isolated Compounds
2.2. Anti-Inflammatory Activity
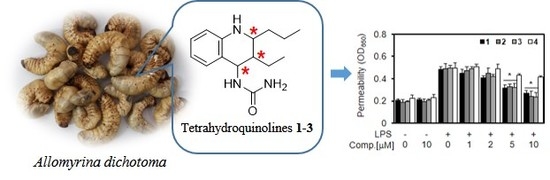
2.2.1. Effect of New Compounds (1–4) on Lipopolysaccharide (LPS)-mediated Vascular Inflammatory Responses
2.2.2. Effect of New Compounds (1–3) on the LPS-stimulated Activation of Nuclear Factor-κB (NF-κB), Production of Interleukin-1β (IL-1β)/Tumor Necrosis Factor-α (TNF-α), and Phosphorylation of p38 MAPK
2.2.3. Protective Effect of Each Compound in Cecal Ligation and Puncture (CLP)-Induced Septic Mice
2.2.4. Quantitative Analysis of 1–3
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Insect Material
3.3. Extraction and Isolation
3.3.1. Allomyrinaine A (1)
3.3.2. Allomyrinaine B (2)
3.3.3. Allomyrinaine C (3)
3.3.4. Allomyrinamide A (4)
3.4. Computational NMR Chemical Shift Calculations
3.5. Biological Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huis, A.V.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects. Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 171. [Google Scholar]
- Song, M.C.; Yang, H.J.; Bang, M.H.; Kim, Y.C.; Kim, S.Y.; Rho, Y.D.; Chung, S.H.; Baek, N.I. Isolation and quantitative analysis of inosine as an index component from larva of Allomirina dichotoma. J. Korean Soc. Appl. Biol. Chem. 2006, 49, 334–338. [Google Scholar]
- Youn, K.; Kim, J.Y.; Yeo, H.; Yun, E.Y.; Hwang, J.S.; Jun, M. Fatty acid and volatile oil compositions of Allomyrina dichotoma larvae. Prev. Nutr. Food Sci. 2012, 17, 310–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.E.; Jo, D.E.; Lee, A.J.; Park, H.K.; Youn, K.; Yun, E.Y.; Hwang, J.S.; Jun, M.; Kang, B.H. Hepatoprotective and anticancer activities of Allomyrina dichotoma larvae. J. Life Sci. 2015, 25, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Youn, K.; Yun, E.-Y.; Hwang, J.S.; Ahn, M.R.; Jeong, W.S.; Jun, M. Effects of solvent fractions of Allomyrina dichotoma larvae through the inhibition of in vitro BACE1 and β-amyloid(25-35)-induced toxicity in rat pheochromocytoma PC12 cells. Entomol. Res. 2014, 44, 23–30. [Google Scholar] [CrossRef]
- Kim, J.; Yun, E.Y.; Park, S.W.; Goo, T.-W.; Seo, M. Allomyrina dichotoma larvae regulate food intake and body weight in high fat diet-induced obese mice through mTOR and Mapk signaling pathways. Nutrients 2016, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Suh, H.J.; Kim, S.R.; Lee, K.S.; Park, S.; Kang, S.C. Antioxidant activity of various solvent extracts from Allomyrina dichotoma (Arthropoda: Insecta) larvae. J. Photochem. Photobiol. B 2010, 99, 67–73. [Google Scholar] [CrossRef]
- Goldblum, S.E.; Brann, T.W.; Ding, X.; Pugin, J.; Tobias, P.S. Lipopolysaccharide (LPS)-binding protein and soluble CD14 function as accessory molecules for LPS-induced changes in endothelial barrier function, in vitro. J. Clin. Investig. 1994, 93, 692–702. [Google Scholar] [CrossRef]
- Varani, J.; Ward, P.A. Mechanisms of endothelial cell injury in acute inflammation. Shock 1994, 2, 311–319. [Google Scholar] [CrossRef]
- Aird, W.C. Endothelium as a therapeutic target in sepsis. Curr. Drug Targets 2007, 8, 501–507. [Google Scholar] [CrossRef]
- Czermak, B.J.; Breckwoldt, M.; Ravage, Z.B.; Huber-Lang, M.; Schmal, H.; Bless, N.M.; Friedl, H.P.; Ward, P.A. Mechanisms of enhanced lung injury during sepsis. Am. J. Pathol. 1999, 154, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Pu, Y.; Kong, W.; Tang, X.; Zhou, J.; Gou, H.; Song, X.; Zhou, H.; Gao, N.; Shen, J. Antidesmone, a unique tetrahydroquinoline alkaloid, prevents acute lung injury via regulating MAPK and NF-κB activities. Int. Immunopharmacol. 2017, 45, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Tuan, N.Q.; Oh, J.; Park, H.B.; Ferreira, D.; Choe, S.; Lee, J.; Na, M. A grayanotox-9(11)-ene derivative from Rhododendron brachycarpum and its structural assignment via a protocol combining NMR and DP4 plus application. Phytochemistry 2017, 133, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, H.; Kim, M.A.; Choi, J.; Kim, K.M.; Hwang, J.S.; Na, M.; Bae, J.S. Evaluation of novel factor Xa inhibitors from Oxya chinensis sinuosa with anti-platelet aggregation activity. Sci. Rep. 2017, 7, 7934–7946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erenler, R.; Sen, O.; Aksit, H.; Demirtas, I.; Yaglioglu, A.S.; Elmastas, M.; Telci, I. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J. Sci. Food Agric. 2016, 96, 822–836. [Google Scholar] [CrossRef]
- Lee, W.; Kim, M.A.; Park, I.; Hwang, J.S.; Na, M.; Bae, J.S. Novel direct factor Xa inhibitory compounds from Tenebrio molitor with anti-platelet aggregation activity. Food Chem. Toxicol. 2017, 109, 19–27. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, I.H.; Kim, J.H.; Kim, M.A.; Hwang, J.S.; Kim, Y.H.; Na, M. Quinoxaline-, dopamine-, and amino acid-derived metabolites from the edible insect Protaetia brevitarsis seulensis. Arch. Pharm. Res. 2017, 40, 1064–1070. [Google Scholar] [CrossRef]
- Riedemann, N.C.; Guo, R.-F.; Ward, P.A. Novel strategies for the treatment of sepsis. Nat. Med. 2003, 9, 517–524. [Google Scholar] [CrossRef]
- Jonathan, C. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar]
- Bierhaus, A.; Chen, J.; Liliensiek, B.; Nawroth, P.P. LPS and cytokine-activated endothelium. Semin. Thromb. Hemost. 2000, 26, 571–587. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.Y.; Yoo, Y.; Kim, S.Y.; Kim, J.E.; Kim, S.W.; Seo, Y.K.; Park, E.K.; Kim, I.S.; Bae, J.S. Macrophagic stabilin-1 restored disruption of vascular integrity caused by sepsis. Thromb. Haemost. 2018, 118, 1776–1789. [Google Scholar] [CrossRef]
- Bae, J.S. Role of high mobility group box 1 in inflammatory disease: Focus on sepsis. Arch. Pharm. Res. 2012, 35, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Tuan, N.Q.; Lee, W.; Oh, J.; Kwak, S.; Lee, H.G.; Ferreira, D.; Bae, J.S.; Na, M. Quinic acid derivatives from Salicornia herbacea alleviate HMGB1-mediated endothelial dysfunction. J. Funct. Foods 2015, 15, 326–338. [Google Scholar] [CrossRef]
- Walton, K.L.; Holt, L.; Sartor, R.B. Lipopolysaccharide activates innate immune responses in murine intestinal myofibroblasts through multiple signaling pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G601–G611. [Google Scholar] [CrossRef] [Green Version]
- Astiz, M.E.; Rackow, E.C. Septic shock. Lancet 1998, 351, 1501–1505. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Bui, B.P.; Oh, Y.; Lee, H.; Cho, J. Inhibition of inflammatory mediators and cell migration by 1,2,3,4-tetrahydroquinoline derivatives in LPS-stimulated BV2 microglial cells via suppression of NF-κB and JNK pathway. Int. Immunopharmacol. 2020, 80, 106231–106241. [Google Scholar] [CrossRef] [PubMed]





| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH, mult (J in Hz) | δC | δH, mult (J in Hz) | δC | δH, mult (J in Hz) | δC | |
| 2 | 3.14, dd (6.54, 11.87) | 52.5 | 3.28, m | 51.1 | 3.21, td (4.14, 7.43) | 54.2 |
| 3 | 1.63, qd (4.24, 6.54) | 42.7 | 1.68, m | 44.2 | 1.67, qd (5.46, 7.43) | 44.3 |
| 4 | 4.99, d, (4.24) | 47.8 | 4.66, d (3.55) | 50.7 | 4.70, d (5.46) | 50.8 |
| 4a | - | 122.5 | - | 121.2 | - | 123.3 |
| 5 | 7.08, d (7.47) | 128.6 | 7.08, dd (1.02, 7.82) | 131.7 | 7.04, d (7.51) | 129.5 |
| 6 | 6.50, m, overlap | 117.0 | 6.56, m, overlap | 117.8 | 6.56, m, overlap | 117.8 |
| 7 | 6.92, m | 128.9 | 6.96, m | 129.1 | 6.93, m | 128.8 |
| 8 | 6.50, m, overlap | 114.7 | 6.56, m, overlap | 115.6 | 6.56, m, overlap | 115.4 |
| 8a | - | 145.5 | - | 146.3 | - | 145.9 |
| 9 | 1.55, m | 38.3 | 1.53, m | 34.8 | 1.62/1.56, m | 37.4 |
| 10 | 1.46, m | 19.4 | 1.49/1.40, m | 20.4 | 1.52, m | 23.4 |
| 11 | 0.97, t (6.40) | 14.6 | 0.99, m, overlap | 14.5 | 0.97, t (7.55), overlap | 14.6 |
| 12 | 1.32, m | 20.7 | 1.44/0.99, m | 19.4 | 1.50/1.43, m | 19.6 |
| 13 | 1.00, t (6.61) | 12.2 | 0.99, m, overlap | 12.4 | 0.97, t (7.55), overlap | 10.5 |
| 15 | - | 161.8 | - | 161.0 | - | 162.2 |
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH, mult (J in Hz) | δC | δH, mult (J in Hz) | δC | δH, mult (J in Hz) | δC | |
| 2 | 3.15, q (6.10, 11.31) | 52.1 | 3.31, brs | 50.6 | 3.24, m | 53.7 |
| 3 | 1.61, qd (4.57, 6.10) | 42.3 | 1.69, td (3.74, 7.87) | 43.8 | 1.66, qd (4.17, 6.72) | 43.7 |
| 4 | 5.08, overlap | 46.9 | 4.71, dd (3.74) | 49.9 | 4.78, t (7.82) | 49.9 |
| 4a | - | 123.1 | - | 121.3 | - | 123.6 |
| 5 | 7.13, d (7.50) | 128.8 | 7.10, d (1.39) | 131.7 | 7.07, d, (7.60) | 129.4 |
| 6 | 6.47, td (1.02, 7.43) | 116.4 | 6.52, td (1.39, 7.78) | 117.1 | 6.50, m | 117.0 |
| 7 | 6.89, m | 128.3 | 6.92, m | 128.5 | 6.89, m | 128.2 |
| 8 | 6.52, d (7.43) | 114.2 | 6.54, d (7.78) | 114.8 | 6.53, d (8.12) | 114.6 |
| 8a | - | 145.1 | - | 146.0 | - | 145.7 |
| 9 | 1.54, m | 37.8 | 1.51, m | 34.8 | 1.62/1.55, m | 37.0 |
| 10 | 1.54/1.45, m | 18.9 | 1.43, m | 18.9 | 1.43, m | 19.2 |
| 11 | 0.93, t (7.18) | 14.5 | 0.94, m, overlap | 14.5 | 0.92, m, overlap | 14.5 |
| 12 | 1.32, m | 20.3 | 1.51/1.38, m | 20.1 | 1.50, m | 23.0 |
| 13 | 0.98, t (7.42) | 12.2 | 0.96, m, overlap | 12.4 | 0.95, m, overlap | 10.4 |
| 15 | - | 159.1 | - | 158.2 | - | 159.5 |
| NH-1 | 5.08, m, overlap | - | 4.96, s | - | 4.88, s | - |
| NH-14 | 5.64, d (9.74) | - | 5.74, d (6.69) | - | 5.59, d (7.78) | - |
| NH2-16 | 5.08, m, overlap | - | 5.01, s | - | 5.09, s | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, I.; Lee, W.; Yoo, Y.; Shin, H.; Oh, J.; Kim, H.; Kim, M.-A.; Hwang, J.S.; Bae, J.-S.; Na, M. Protective Effect of Tetrahydroquinolines from the Edible Insect Allomyrina dichotoma on LPS-Induced Vascular Inflammatory Responses. Int. J. Mol. Sci. 2020, 21, 3406. https://doi.org/10.3390/ijms21103406
Park I, Lee W, Yoo Y, Shin H, Oh J, Kim H, Kim M-A, Hwang JS, Bae J-S, Na M. Protective Effect of Tetrahydroquinolines from the Edible Insect Allomyrina dichotoma on LPS-Induced Vascular Inflammatory Responses. International Journal of Molecular Sciences. 2020; 21(10):3406. https://doi.org/10.3390/ijms21103406
Chicago/Turabian StylePark, InWha, Wonhwa Lee, Youngbum Yoo, Hyosoo Shin, Joonseok Oh, Hyelim Kim, Mi-Ae Kim, Jae Sam Hwang, Jong-Sup Bae, and MinKyun Na. 2020. "Protective Effect of Tetrahydroquinolines from the Edible Insect Allomyrina dichotoma on LPS-Induced Vascular Inflammatory Responses" International Journal of Molecular Sciences 21, no. 10: 3406. https://doi.org/10.3390/ijms21103406