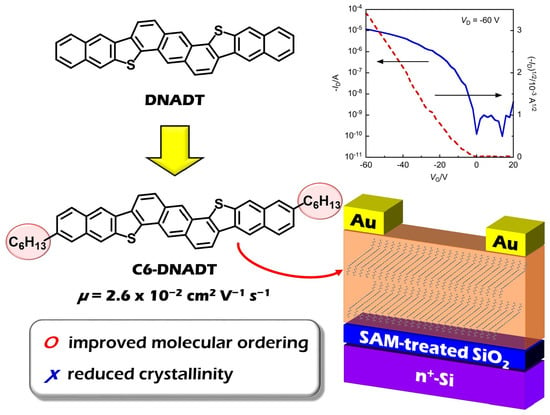
Synthesis of Dinaphtho[2,3-d:2’,3’-d’]anthra[1,2-b:5,6-b’]dithiophene (DNADT) Derivatives: Effect of Alkyl Chains on Transistor Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Theoretical Calculations for Molecular Design
2.2. Synthesis of C6-DNADT
2.3. Physicochemical Properties of C6-DNADT
2.3.1. UV-Vis Absorption Spectrum and Cyclic Voltammogram
2.3.2. Thermal Stability
2.3.3. OFET Properties
2.3.4. AFM Images
2.3.5. GIWAXS Images
3. Materials and Methods
3.1. Instrumentation
3.2. Chemicals
3.3. Experimental Procedures
3.3.1. Synthesis of 2-Hexyl-6-methoxynaphthalene (2)
3.3.2. Synthesis of 3-Bromo-6-hexyl-2-methoxynaphthalene (3)
3.3.3. Synthesis of 3-Bromo-6-hexylnaphthalen-2-ol (4)
3.3.4. Synthesis of 3-Bromo-6-hexyl-2-(trifluoromethanesulfonyloxy)naphthalene (5)
3.3.5. Synthesis of 3-Bromo-6-hexyl-2-(2-trimethylsilylethynyl)naphthalene (6)
3.3.6. Synthesis of 6-Hexylnaphtho[2,3-b]thiophene (7)
3.3.7. Synthesis of 2,5-Bis(7-hexylnaphtho[2,3-b]thiophen-2-yl)benzendicarboxaldehyde (9)
3.3.8. Synthesis of 2,2′-(2,5-Bis(7-hexylnaphtho[2,3-b]thiophen-2-yl)-1,4-phenylene)bis(oxirane) (10)
3.3.9. Synthesis of 4,14-dihexyldinaphto[2,3-d:2′,3′-d’]anthra[1,2-b:5,6-b’]dithiophene (C6-DNADT)
3.4. Fabrication of Vapor-Deposited OFET Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OFET | Organic field-effect transistor |
| C6-DNADT | 4,14-Dihexyldinaphtho[2,3-d:2’,3’-d’]anthra[1,2-b:5,6-b’]dithiophene |
| GIWAXS | Grazing incidence wide-angle X-ray scattering |
| AFM | Atomic force microscopy |
| HOMO | Highest occupied molecular orbital |
| C8-BTBT | 2,7-Dioctyl[1]benzothieno[3,2-b][1]benzothiophene |
| XRD | X-ray diffraction |
| DFT | Density functional theory |
| NHOMO | Second (next) highest occupied molecular orbital |
| DMF | N,N-Dimethylformamide |
| dppf | 1,1’-Bis(diphenylphosphino)ferrocene |
| THF | Tetrahydrofuran |
| TMS | Trimethylsilyl |
| DCE | 1,2-Dichloroethane |
| NMP | N-Methyl-2-pyrrolidone |
| NMR | Nuclear magnetic resonance |
| UV | Ultraviolet |
| TGA | Thermogravimetric analysis |
| DSC | Differential scanning calorimetry |
| OTS | n-Octyltrichlorosilane |
| ODTS | n-Octadecyltrichlorosilane |
| SAM | Self-assembled monolayer |
| RMS | Root-mean-square |
| TLC | Thin layer chromatography |
| HRMS | High-resolution mass spectrometry |
| FT-IR | Fourier transform infrared spectroscopy |
| TCI | Tokyo Chemical Industry Co., Ltd. |
| FAB | Fast atom bombardment |
| EI | Electron impact |
| equiv | Equivalent |
| sat. | Saturated |
| aq. | Aqueous |
References
- Mori, T.; Nishimura, T.; Yamamoto, T.; Doi, I.; Miyazaki, E.; Osaka, I.; Takimiya, K. Consecutive Thiophene-Annulation Approach to π-Extended Thienoacene-Based Organic Semiconductors with [1]Benzothieno[3,2-b][1]benzothiophene (BTBT) Substructure. J. Am. Chem. Soc. 2013, 135, 13900–13913. [Google Scholar] [CrossRef]
- Mitsui, C.; Okamoto, T.; Yamagishi, M.; Tsurumi, J.; Yoshimoto, K.; Nakahara, K.; Soeda, J.; Hirose, Y.; Sato, H.; Yamano, A.; et al. High-Performance Solution-Processable N-Shaped Organic Semiconducting Materials with Stabilized Crystal Phase. Adv. Mater. 2014, 26, 4546–4551. [Google Scholar] [CrossRef]
- Okamoto, H.; Eguchi, R.; Hamao, S.; Goto, H.; Sakai, Y.; Izumi, M.; Takaguchi, Y.; Gohda, S.; Kubozono, Y. An Extended Phenacene-type Molecule, [8]Phenacene: Synthesis and Transistor Application. Sci. Rep. 2014, 4, 5330. [Google Scholar] [CrossRef]
- Abe, M.; Mori, T.; Osaka, I.; Sugimoto, K.; Takimiya, K. Thermally, Operationally, and Environmentally Stable Organic Thin-Film Transistors Based on Bis[1]benzothieno[2,3-d:2′,3′-d′]naphtho[2,3-b:6,7-b′]dithiophene Derivatives: Effective Synthesis, Electronic Structures, and Structure–Property Relationship. Chem. Mater. 2015, 27, 5049–5057. [Google Scholar] [CrossRef]
- Yamamoto, A.; Murata, Y.; Mitsui, C.; Ishii, H.; Yamagishi, M.; Yano, M.; Sato, H.; Yamano, A.; Takeya, J.; Okamoto, T. Zigzag-Elongated Fused π-Electronic Core: A Molecular Design Strategy to Maximize Charge-Carrier Mobility. Adv. Sci. 2018, 5, 1700317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeya, J.; Yamagishi, M.; Tominari, Y.; Hirahara, R.; Nakazawa, Y.; Nishikawa, T.; Kawase, T.; Shimoda, T.; Ogawa, S. Very high-mobility organic single-crystal transistors with in-crystal conduction channels. Appl. Phys. Lett. 2007, 90, 102120. [Google Scholar] [CrossRef]
- Kanashima, T.; Katsura, Y.; Okuyama, M. Organic ferroelectric gate field-effect transistor memory using high-mobility rubrene thin film. Jpn. J. Appl. Phys. 2014, 53, 04ED11. [Google Scholar] [CrossRef]
- Yuan, Y.; Giri, G.; Ayzner, A.L.; Zoombelt, A.P.; Mannsfeld, S.C.B.; Chen, J.; Nordlund, D.; Toney, M.F.; Huang, J.; Bao, Z. Ultra-high mobility transparent organic thin film transistors grown by an off-centre spin-coating method. Nat. Commun. 2014, 5, 3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niimi, K.; Shinamura, S.; Osaka, I.; Miyazaki, E.; Takimiya, K. Dianthra[2,3-b:2′,3′-f]thieno[3,2-b]thiophene (DATT): Synthesis, Characterization, and FET Characteristics of New π-Extended Heteroarene with Eight Fused Aromatic Rings. J. Am. Chem. Soc. 2011, 133, 8732–8739. [Google Scholar] [CrossRef]
- Mitsui, C.; Yamagishi, M.; Shikata, R.; Ishii, H.; Matsushita, T.; Nakahara, K.; Yano, M.; Sato, H.; Yamano, A.; Takeya, J.; et al. Oxygen- and Sulfur-Bridged Bianthracene V-Shaped Organic Semiconductors. Bull. Chem. Soc. Jpn. 2017, 90, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubozono, Y.; He, X.; Hamao, S.; Teranishi, K.; Goto, H.; Eguchi, R.; Kambe, T.; Gohda, S.; Nishihara, Y. Transistor Application of Phenacene Molecules and Their Characteristics. Eur. J. Inorg. Chem. 2014, 2014, 3806–3819. [Google Scholar] [CrossRef]
- Yasuda, T.; Goto, T.; Fujita, K.; Tsutsui, T. Ambipolar pentacene field-effect transistors with calcium source-drain electrodes. Appl. Phys. Lett. 2004, 85, 2098–2100. [Google Scholar] [CrossRef]
- Inokuchi, H.; Saito, G.; Wu, P.; Seki, K.; Tang, T.B.; Mori, T.; Imaeda, K.; Enoki, T.; Higuchi, Y.; Inaka, K.; et al. A Novel Type of Organic Semiconductors. Molecular Fastener. Chem. Lett. 1986, 15, 1263–1266. [Google Scholar] [CrossRef]
- Okamoto, H.; Hamao, S.; Goto, H.; Sakai, Y.; Izumi, M.; Gohda, S.; Kubozono, Y.; Eguchi, R. Transistor application of alkyl-substituted picene. Sci. Rep. 2014, 4, 5048. [Google Scholar] [CrossRef] [Green Version]
- Ebata, H.; Izawa, T.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H.; Yui, T.J. Highly Soluble [1]Benzothieno[3,2-b]benzothiophene (BTBT) Derivatives for High-Performance, Solution-Processed Organic Field-Effect Transistors. J. Am. Chem. Soc. 2007, 129, 15732–15733. [Google Scholar] [CrossRef]
- Kang, M.J.; Doi, I.; Mori, H.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H. Alkylated Dinaphtho[2,3-b:2′,3′-f]Thieno[3,2-b]Thiophenes (Cn-DNTTs): Organic Semiconductors for High-Performance Thin-Film Transistors. Adv. Mater. 2011, 23, 1222–1225. [Google Scholar] [CrossRef]
- Hyodo, K.; Nonobe, H.; Nishinaga, S.; Nishihara, Y. Synthesis of 2,9-dialkylated phenanthro[1,2-b:8,7-b’]dithiophenes via cross-coupling reactions and sequential Lewis acid-catalyzed regioselective cycloaromatization of epoxide. Tetrahedron Lett. 2014, 55, 4002–4005. [Google Scholar] [CrossRef]
- Kubozono, Y.; Hyodo, K.; Mori, H.; Hamao, S.; Goto, H.; Nishihara, Y. Transistor application of new picene-type molecules, 2,9-dialkylated phenanthro[1,2-b:8,7-b’]dithiophenes. J. Mater. Chem. C 2015, 3, 2413–2421. [Google Scholar] [CrossRef]
- Xiong, Y.; Qiao, X.; Wu, H.; Huang, Q.; Wu, Q.; Li, J.; Gao, X.; Li, H. Synthesis and Properties of Nine-Ring-Fused Linear Thienoacenes. J. Org. Chem. 2014, 79, 1138–1144. [Google Scholar] [CrossRef]
- Chang, N.; Chen, X.; Nonobe, H.; Okuda, Y.; Mori, H.; Nakajima, K.; Nishihara, Y. Synthesis of Substituted Picenes through Pd-Catalyzed Cross-Coupling Reaction/Annulation Sequences and Their Physicochemical Properties. Org. Lett. 2013, 15, 3558–3561. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Mori, H.; Chen, X.; Okuda, Y.; Okamoto, T.; Nishihara, Y. Synthesis of Substituted [6]Phenacenes through Suzuki-Miyaura Coupling of Polyhalobenzene with Alkenylboronates and Sequential Intramolecular Cyclization via C-H Bond Activation. Chem. Lett. 2013, 42, 1257–1259. [Google Scholar] [CrossRef] [Green Version]
- Nishihara, Y.; Kinoshita, M.; Hyodo, K.; Okuda, Y.; Eguchi, R.; Goto, H.; Hamao, S.; Takabayashi, Y.; Kubozono, Y. Phenanthro[1,2-b:8,7-b’]dithiophene: A New Picene-type Molecule for Transistor Applications. RSC Adv. 2013, 3, 19341–19347. [Google Scholar] [CrossRef]
- Mori, H.; Chen, X.; Chang, N.; Hamao, S.; Kubozono, Y.; Nakajima, K.; Nishihara, Y. Synthesis of Methoxy-Substituted Picenes: Substitution Position Effect on Their Electronic and Single-Crystal Structures. J. Org. Chem. 2014, 79, 4973–4983. [Google Scholar] [CrossRef]
- Chen, X.; Nishinaga, S.; Okuda, Y.; Zhao, J.; Xu, J.; Mori, H.; Nishihara, Y. A Divergent Synthesis of 3,10-Dialkylpicenes. Org. Chem. Front. 2015, 3, 536–541. [Google Scholar] [CrossRef]
- Kubozono, Y.; Hyodo, K.; Hamao, S.; Shimo, Y.; Mori, H.; Nishihara, Y. Transistor Properties of 2,7-Dialkyl-Substituted Phenanthro[2,1-b:7,8-b’]dithiophene. Sci. Rep. 2016, 6, 38535. [Google Scholar] [CrossRef] [Green Version]
- Hyodo, K.; Hagiwara, H.; Toyama, R.; Mori, H.; Soga, S.-I.; Nishihara, Y. Bis[1]benzothieno[2,3-d:2’,3’-d’]anthra[1,2-b:5,6-b’]dithiophene: Synthesis, characterization, and application to organic field-effect transistors. RSC Adv. 2017, 7, 6089–6092. [Google Scholar] [CrossRef] [Green Version]
- Hyodo, K.; Toyama, R.; Mori, H.; Nishihara, Y. Synthesis and Physicochemical Properties of Piceno[4,3-b:9,10-b’]dithiophene Derivatives and Their Application in Organic Field-Effect Transistors. ACS Omega 2017, 2, 308–315. [Google Scholar] [CrossRef]
- Nishinaga, S.; Mori, H.; Nishihara, Y. Synthesis and Transistor Application of Bis[1]benzothieno[6,7-d:6’,7’-d’]benzo[1,2-b:4,5-b’]dithiophenes. J. Org. Chem. 2018, 83, 5506–5515. [Google Scholar] [CrossRef]
- Hyodo, K.; Nishinaga, S.; Sawanaka, Y.; Ishida, T.; Mori, H.; Nishihara, Y. Synthesis and Physicochemical Properties of Dibenzo[2,3-d:2’,3’-d’]anthra[1,2-b:5,6-b’]dithiophene (DBADT) and Its Derivatives: Effect of Substituents on Their Molecular Orientation and Transistor Properties. J. Org. Chem. 2019, 84, 698–709. [Google Scholar] [CrossRef]
- Nishinaga, S.; Mitani, M.; Mori, H.; Okamoto, T.; Takeya, J.; Nishihara, Y. Bis[1]benzothieno[5,4-d:5’,4’-d’]benzo[1,2-b:4,5-b’]dithiophene Derivatives: Synthesis and Effect of Sulfur Positions on Their Transistor Properties. Bull. Chem. Soc. Jpn. 2019, 92, 1107–1116. [Google Scholar] [CrossRef]
- Nishinaga, S.; Sawanaka, Y.; Toyama, R.; Ishida, T.; Mori, H.; Nishihara, Y. Synthesis and Transistor Characteristics of Dinaphtho[2,3-d:2′,3′-d’]anthra[1,2-b:5,6-b’]dithiophene (DNADT). Chem. Lett. 2018, 47, 1409–1411. [Google Scholar] [CrossRef]
- Niimi, K.; Kang, M.J.; Miyazaki, E.; Osaka, I.; Takimiya, K. General Synthesis of Dinaphtho[2,3-b:2′,3′-f]thieno[3,2-b]thiophene (DNTT) Derivatives. Org. Lett. 2011, 13, 3430–3433. [Google Scholar] [CrossRef]
- Okamoto, T.; Mitsui, C.; Yamagishi, M.; Nakahara, K.; Soeda, J.; Hirose, Y.; Miwa, K.; Sato, H.; Yamano, A.; Matsushita, T.; et al. V-Shaped Organic Semiconductors with Solution Processability, High Mobility, and High Thermal Durability. Adv. Mater. 2013, 25, 6392–6397. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Yi, W.; Zhao, S.; Sun, H.; Kan, Y.; Shi, J.; Wan, S.; Lia, C.; Wang, S. Isomers of organic semiconductors based on dithienothiophenes: The effect of sulphur atoms positions on the intermolecular interactions and field-effect performances. J. Mater. Chem. C 2015, 3, 10856–10861. [Google Scholar] [CrossRef]
- Mas-Torrent, M.; Durkut, M.; Hadley, P.; Ribas, X.; Rovira, C. High Mobility of Dithiophene-Tetrathiafulvalene Single-Crystal Organic Field Effect Transistors. J. Am. Chem. Soc. 2004, 126, 984–985. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Li, Y.; Wang, Z. Heteroarenes as high performance organic semiconductors. Chem. Soc. Rev. 2013, 42, 6113–6127. [Google Scholar] [CrossRef]
- Kera, S.; Yamane, H.; Ueno, N. First-principles measurements of charge mobility in organic semiconductors: Valence hole–vibration coupling in organic ultrathin film. Prog. Surf. Sci. 2009, 84, 135–154. [Google Scholar] [CrossRef]
- Ishii, H.; Kobayashi, N.; Hirose, K. Carrier transport calculations of organic semiconductors with static and dynamic disorder. Jpn. J. Appl. Phys. 2019, 58, 110501. [Google Scholar] [CrossRef]
- Niimi, K.; Miyazaki, E.; Osaka, I.; Takimiya, K. Facile Syntheses of Anthra[2,3-b]chalcogenophenes. Synthesis 2012, 44, 2102–2106. [Google Scholar]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Sasaki, M.; Nomoto, K. Stable peri-Xanthenoxanthene Thin-Film Transistors with Efficient Carrier Injection. Chem. Mater. 2009, 21, 552–556. [Google Scholar] [CrossRef]
- Kang, B.; Jang, M.; Chung, Y.; Kim, H.; Kwak, S.K.; Oh, J.H.; Cho, K. Enhancing 2D growth of organic semiconductor thin films with macroporous structures via a small-molecule heterointerface. Nat. Commun. 2014, 5, 4752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minemawari, H.; Tanaka, M.; Tsuzuki, S.; Inoue, S.; Yamada, T.; Kumai, R.; Shimoi, Y.; Hasegawa, T. Enhanced Layered-Herringbone Packing due to Long Alkyl Chain Substitution in Solution-Processable Organic Semiconductors. Chem. Mater. 2017, 29, 1245–1254. [Google Scholar] [CrossRef]
- Seo, S.; Marks, T.J. Lanthanide-Catalyst-Mediated Tandem Double Intramolecular Hydroalkoxylation/Cyclization of Dialkynyl Dialcohols: Scope and Mechanism. Chem. Eur. J. 2010, 16, 5148–5162. [Google Scholar] [CrossRef]








| SAM | Tanneal / °C a | µmax / cm2 V–1 s–1 b | Vth/V | Ion/Ioff |
|---|---|---|---|---|
| OTS | as depo. | 1.9 × 10−2 | −9 | 104–105 |
| 50 | 2.1 × 10−2 | −13 | 104–105 | |
| 100 | 2.6 × 10−2 | −10 | 104–105 | |
| 150 | 2.1 × 10−2 | −12 | 104–105 | |
| ODTS | as depo. | 1.4 × 10−2 | −22 | 104–105 |
| 50 | 7.7 × 10−3 | −5 | 104–105 | |
| 100 | 6.9 × 10−3 | −5 | 104–105 | |
| 150 | 4.4 × 10−3 | −2 | 104–105 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, T.; Sawanaka, Y.; Toyama, R.; Ji, Z.; Mori, H.; Nishihara, Y. Synthesis of Dinaphtho[2,3-d:2’,3’-d’]anthra[1,2-b:5,6-b’]dithiophene (DNADT) Derivatives: Effect of Alkyl Chains on Transistor Properties. Int. J. Mol. Sci. 2020, 21, 2447. https://doi.org/10.3390/ijms21072447
Ishida T, Sawanaka Y, Toyama R, Ji Z, Mori H, Nishihara Y. Synthesis of Dinaphtho[2,3-d:2’,3’-d’]anthra[1,2-b:5,6-b’]dithiophene (DNADT) Derivatives: Effect of Alkyl Chains on Transistor Properties. International Journal of Molecular Sciences. 2020; 21(7):2447. https://doi.org/10.3390/ijms21072447
Chicago/Turabian StyleIshida, Takumi, Yuta Sawanaka, Ryota Toyama, Zhenfei Ji, Hiroki Mori, and Yasushi Nishihara. 2020. "Synthesis of Dinaphtho[2,3-d:2’,3’-d’]anthra[1,2-b:5,6-b’]dithiophene (DNADT) Derivatives: Effect of Alkyl Chains on Transistor Properties" International Journal of Molecular Sciences 21, no. 7: 2447. https://doi.org/10.3390/ijms21072447