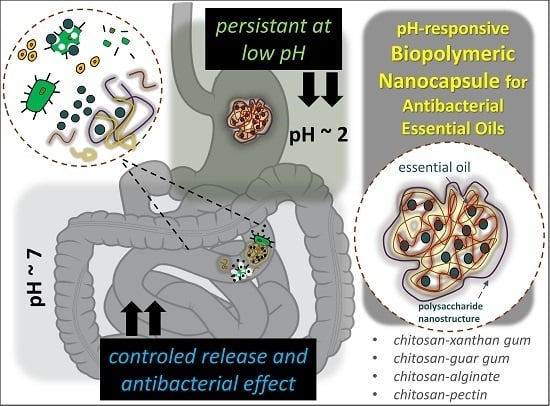
Development of pH-Responsive Biopolymeric Nanocapsule for Antibacterial Essential Oils
Abstract
:1. Introduction
2. Results
2.1. Effect of TPP and Tween 80 on Nanocapsules EE and LE
2.2. EOs Release from NCaps under Physiologic Conditions
2.3. Inhibition Activity on Eucaryotic Model and Blood Compatibility Assessment of NCaps
2.4. Inhibition Effect of NCaps on G+ and G− Pathogenic Bacteria
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Biopolymeric EOs-NCaps Preparation
4.3. Folin-Ciocalteau Reaction
4.4. Ninyhdrin Assay for Chitosan Detection
4.5. Absorbance Measurements
4.6. Encapsulation Efficiency and Loading Efficiency Calculation
4.7. Nanocapsule Size and ζ-potential Measurement
4.8. The Cumulative Release of EOs
(encapsulated EOs concentration/released EOs concentration) × 100
4.9. Gas Chromatography with Flame Ionisation Detection
4.10. Minimal Inhibition Concentration Determination
4.11. Viability Testing
((log10 number of cells − log10 number of cells) 2.303)/(time1 − time0)
4.12. Haemolytic Assay
4.13. Data Treatment and Descriptive Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A | Alginate |
| C | Chitosan |
| C-A NCaps | Chitosan-alginate nanocapsules |
| C-G NCaps | Chitosan-Guar gum nanocapsules |
| C-NCaps | Chitosan nanocapsules |
| C-P NCaps | Chitosan-pectin nanocapsules |
| C-X NCaps | Chitosan-xanthan nanocapsules |
| DMSO | Dimethyl sulfoxide |
| EE | encapsulation efficiency |
| EOs | Essential oils |
| FC | Folin-Ciocalteau |
| G | Guar gum |
| LC | loading efficiency |
| MH | Müller–Hinton medium |
| NCaps | Nanocapsules |
| P | Pectin |
| PBS | Phosphate buffer saline |
| RBC | Red blood cells |
| R-EOs | Rosmarinus officinalis EOs |
| SDS | Sodium dodecyl sulphate |
| S-EOs | Syzygium aromaticum EOs |
| T-EOs | Thymus vulgaris EOs |
| TPP | Tripolyphosphate penta basic |
| X | Xanthan gum |
References
- De Briyne, N.; Atkinson, J.; Pokludova, L.; Borriello, S.P. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175. [Google Scholar] [CrossRef] [Green Version]
- Orhan-Yanikan, E.; da Silva-Janeiro, S.; Ruiz-Rico, M.; Jimenez-Belenguer, A.I.; Ayhan, K.; Barat, J.M. Essential oils compounds as antimicrobial and antibiofilm agents against strains present in the meat industry. Food Control 2019, 101, 29–38. [Google Scholar] [CrossRef]
- Horky, P.; Skalickova, S.; Smerkova, K.; Skladanka, J. Essential Oils as a Feed Additives: Pharmacokinetics and Potential Toxicity in Monogastric Animals. Animals 2019, 9, 352. [Google Scholar] [CrossRef] [Green Version]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Jalilzadeh-Amin, G.; Qarehdarvishlu, B.M. Effects of Artemisia dracunculus essential oil on diarrhea and intestinal transit time in rat gastrointestinal tract. Physiol. Pharmacol. 2015, 18, 416–428. [Google Scholar]
- Volic, M.; Pajic-Lijakovic, I.; Djordjevic, V.; Knezevic-Jugovic, Z.; Pecinar, I.; Stevanovic-Dajic, Z.; Veljovic, D.; Hadnadjev, M.; Bugarski, B. Alginate/soy protein system for essential oil encapsulation with intestinal delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef]
- Naskar, S.; Koutsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef]
- Zhang, L.; Sang, Y.; Feng, J.; Li, Z.M.; Zhao, A.L. Polysaccharide-based micro/nanocarriers for oral colon-targeted drug delivery. J. Drug Target. 2016, 24, 579–589. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr. Polym. 2020, 227. [Google Scholar] [CrossRef]
- Horky, P.; Skladanka, J.; Nevrkla, P.; Slama, P. Effect of diet supplemented with antioxidants (selenium, copper, vitamins e and c) on antioxidant status and ejaculate quality of breeding boars. Ann. Anim. Sci. 2016, 16, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Skalickova, S.; Loffelmann, M.; Gargulak, M.; Kepinska, M.; Docekalova, M.; Uhlirova, D.; Stankova, M.; Fernandez, C.; Milnerowicz, H.; Ruttkay-Nedecky, B.; et al. Zinc-Modified Nanotransporter of Doxorubicin for Targeted Prostate Cancer Delivery. Nanomaterials 2017, 7, 435. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.T.; Yilmaz, A.; Akman, P.K.; Bozkurt, F.; Dertli, E.; Basahel, A.; Al-Sasi, B.; Taylan, O.; Sagdic, O. Electrospraying method for fabrication of essential oil loaded-chitosan nanoparticle delivery systems characterized by molecular, thermal, morphological and antifungal properties. Innov. Food Sci. Emerg. Technol. 2019, 52, 166–178. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Taktak, N.E.M.; Awad, O.M.; Elfiki, S.A.; Abou El-Ela, N.E. Preparation and Characterization of Biopolymers Chitosan/Alginate/Gelatin Gel Spheres Crosslinked by Glutaraldehyde. J. Macromol. Sci. Part B-Phys. 2017, 56, 359–372. [Google Scholar] [CrossRef]
- Jesser, E.; Lorenzetti, A.S.; Yeguerman, C.; Murray, A.P.; Domini, C.; Werdin-Gonzalez, J.O. Ultrasound assisted formation of essential oil nanoemulsions: Emerging alternative for Culex pipiens pipiens Say (Diptera: Culicidae) and Plodia interpunctella Hubner (Lepidoptera: Pyralidae) management. Ultrason. Sonochemistry 2020, 61. [Google Scholar] [CrossRef]
- Fareez, I.M.; Lim, S.M.; Zulkefli, N.A.A.; Mishra, R.K.; Ramasamy, K. Cellulose Derivatives Enhanced Stability of Alginate-Based Beads Loaded with Lactobacillus plantarum LAB12 against Low pH, High Temperature and Prolonged Storage. Probiotics Antimicrob. Proteins 2018, 10, 543–557. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Zhang, R.J.; Zou, L.Q.; McClements, D.J. Protein encapsulation in alginate hydrogel beads: Effect of pH on microgel stability, protein retention and protein release. Food Hydrocoll. 2016, 58, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Sriprablom, J.; Luangpituksa, P.; Wongkongkatep, J.; Pongtharangkul, T.; Suphantharika, M. Influence of pH and ionic strength on the physical and rheological properties and stability of whey protein stabilized o/w emulsions containing xanthan gum. J. Food Eng. 2019, 242, 141–152. [Google Scholar] [CrossRef]
- You, R.R.; Xiao, C.M.; Zhang, L.; Dong, Y.R. Versatile particles from water-soluble chitosan and sodium alginate for loading toxic or bioactive substance. Int. J. Biol. Macromol. 2015, 79, 498–503. [Google Scholar] [CrossRef]
- Kulkarni, N.; Wakte, P.; Naik, J. Development of floating chitosan-xanthan beads for oral controlled release of glipizide. Int. J. Pharm. Investig. 2015, 5, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Petri, D.F.S. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydr. Polym. 2013, 92, 1091–1099. [Google Scholar] [CrossRef] [Green Version]
- Kar, R.; Mohapatra, S.; Bhanja, S.; Das, D.; Barik, B. Formulation and In Vitro Characterization of Xanthan Gum-Based Sustained Release Matrix Tables of Isosorbide-5-Mononitrate. Iran. J. Pharm. Res. 2010, 9, 13–19. [Google Scholar]
- Kowalski, G.; Kijowska, K.; Witczak, M.; Kuterasinski, L.; Lukasiewicz, M. Synthesis and Effect of Structure on Swelling Properties of Hydrogels Based on High Methylated Pectin and Acrylic Polymers. Polymers 2019, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Dukovski, B.J.; Mrak, L.; Winnicka, K.; Szekalska, M.; Juretic, M.; Filipovic-Grcic, J.; Pepic, I.; Lovric, J.; Hafner, A. Spray-dried nanoparticle-loaded pectin microspheres for dexamethasone nasal delivery. Dry. Technol. 2019, 37, 1915–1925. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J. Yeast Systems Biology: Model Organism and Cell Factory. Biotechnol. J. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Kunicka-Styczynska, A. Activity of essential oils against food-spoiling yeast. A review. Flavour Fragr. J. 2011, 26, 326–328. [Google Scholar] [CrossRef]
- Konuk, H.B.; Erguden, B. Antifungal activity of various essential oils against Saccharomyces cerevisiae depends on disruption of cell membrane integrity. Biocell 2017, 41, 13–18. [Google Scholar] [CrossRef]
- Zakrzewska, A.; Boorsma, A.; Brul, S.; Hellingwerf, K.J.; Klis, F.M. Transcriptional response of Saccharomyces cerevisiae to the plasma membrane-perturbing compound chitosan. Eukaryot. Cell 2005, 4, 703–715. [Google Scholar] [CrossRef] [Green Version]
- Elmaci, S.B.; Gulgor, G.; Tokatli, M.; Erten, H.; Isci, A.; Ozcelik, F. Effectiveness of chitosan against wine-related microorganisms. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 107, 675–686. [Google Scholar] [CrossRef]
- Baker, L.G.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 855–867. [Google Scholar] [CrossRef] [Green Version]
- de Lima, J.M.; Sarmento, R.R.; de Souza, J.R.; Brayner, F.A.; Feitosa, A.P.S.; Padilha, R.; Alves, L.C.; Porto, I.J.; Batista, R.; de Oliveira, J.E.; et al. Evaluation of Hemagglutination Activity of Chitosan Nanoparticles Using Human Erythrocytes. Biomed Res. Int. 2015. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.S.; Zhou, J.J.; Li, L. An investigation of chitosan and its derivatives on red blood cell agglutination. Rsc Adv. 2017, 7, 12247–12254. [Google Scholar] [CrossRef] [Green Version]
- van Vuuren, S.F.; du Toit, L.C.; Parry, A.; Pillay, V.; Choonara, Y.E. Encapsulation of Essential Oils within a Polymeric Liposomal Formulation for Enhancement of Antimicrobial Efficacy. Nat. Prod. Commun. 2010, 5, 1401–1408. [Google Scholar] [CrossRef] [Green Version]
- Sotelo-Boyas, M.; Correa-Pacheco, Z.; Bautista-Banos, S.; Gomez, Y.G.Y. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef]
- Jacumazo, J.; de Carvalho, M.M.; Parchen, G.P.; Campos, I.M.F.; Garcia, M.J.B.; Brugnari, T.; Maciel, G.M.; Marques, F.A.; de Freitas, R.A. Development, characterization and antimicrobial activity of sodium dodecyl sulfate-polysaccharides capsules containing eugenol. Carbohydr. Polym. 2020, 230. [Google Scholar] [CrossRef]
- Zhaveh, S.; Mohsenifar, A.; Beiki, M.; Khalili, S.T.; Abdollahi, A.; Rahmani-Cherati, T.; Tabatabaei, M. Encapsulation of Cuminum cyminum essential oils in chitosan-caffeic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crop. Prod. 2015, 69, 251–256. [Google Scholar] [CrossRef]
- Bozin, B.; Mlmica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- de Carvalho, F.G.; Magalhaes, T.C.; Teixeira, N.M.; Gondim, B.L.C.; Carlo, H.L.; dos Santos, R.L.; de Oliveira, A.R.; Denadai, A.M.L. Synthesis and characterization of TPP/chitosan nanoparticles: Colloidal mechanism of reaction and antifungal effect on C. albicans biofilm formation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 104. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Prochazkova, S.; Varum, K.M.; Ostgaard, K. Quantitative determination of chitosans by ninhydrin. Carbohydr. Polym. 1999, 38, 115–122. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalickova, S.; Aulichova, T.; Venusova, E.; Skladanka, J.; Horky, P. Development of pH-Responsive Biopolymeric Nanocapsule for Antibacterial Essential Oils. Int. J. Mol. Sci. 2020, 21, 1799. https://doi.org/10.3390/ijms21051799
Skalickova S, Aulichova T, Venusova E, Skladanka J, Horky P. Development of pH-Responsive Biopolymeric Nanocapsule for Antibacterial Essential Oils. International Journal of Molecular Sciences. 2020; 21(5):1799. https://doi.org/10.3390/ijms21051799
Chicago/Turabian StyleSkalickova, Sylvie, Tereza Aulichova, Eva Venusova, Jiri Skladanka, and Pavel Horky. 2020. "Development of pH-Responsive Biopolymeric Nanocapsule for Antibacterial Essential Oils" International Journal of Molecular Sciences 21, no. 5: 1799. https://doi.org/10.3390/ijms21051799