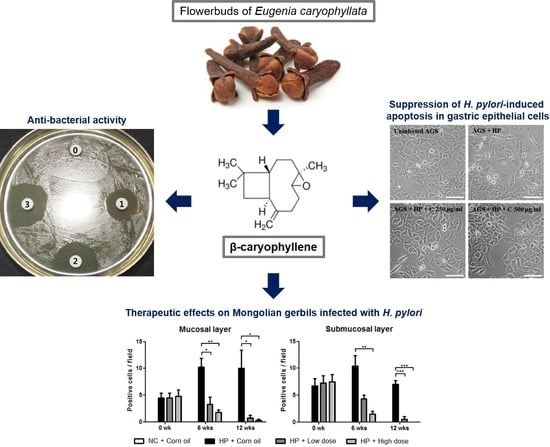
Inhibitory Effects of β-Caryophyllene on Helicobacter pylori Infection In Vitro and In Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibitory Effect of β-Caryophyllene on the Growth and Expression of Replication Genes of H. pylori
2.2. Suppression of H. pylori-Induced Apoptosis in Gastric Epithelial Cells by β-Caryophyllene
2.3. Inhibitory Effects of β-Caryophyllene on the Translocation of Bacterial CagA and VacA Proteins
2.4. Therapeutic Efefcts of β-Caryophyllene on Mongolian Gerbils Infected With H. pylori
2.5. Inhibitory Effects of β-Caryophyllene on the H. pylori-Induced Inflammation
3. Materials and Methods
3.1. Bacterial Culture and Determination of Antibacterial Activity
3.2. Mammalian Cell Culture
3.3. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
3.4. Western Blotting
3.5. WST Cell Viability Assay Using EZ-Cytox
3.6. Annexin V and PI Staining
3.7. Animal and Experimental Design
3.8. Assessment of Histopathology
3.9. Statistical Analysis
3.10. Ethics Statement
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar] [PubMed]
- Vohlonen, I.; Pukkala, E.; Malila, N.; Harkonen, M.; Hakama, M.; Koistinen, V.; Sipponen, P. Risk of gastric cancer in Helicobacter pylori infection in a 15-year follow-up. Scand. J. Gastroenterol. 2016, 51, 1159–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC Working Group. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 1–241. [Google Scholar]
- Backert, S.; Tegtmeyer, N. Type IV Secretion and Signal Transduction of Helicobacter pylori CagA through Interactions with Host Cell Receptors. Toxins (Basel) 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Kwok, T.; Zabler, D.; Urman, S.; Rohde, M.; Hartig, R.; Wessler, S.; Misselwitz, R.; Berger, J.; Sewald, N.; Konig, W.; et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 2007, 449, 862–866. [Google Scholar] [CrossRef]
- Merino, E.; Flores-Encarnacion, M.; Aguilar-Gutierrez, G.R. Functional interaction and structural characteristics of unique components of Helicobacter pylori T4SS. FEBS J. 2017. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.S.; Tegtmeyer, N.; Traube, L.; Jindal, S.; Perez-Perez, G.; Sticht, H.; Backert, S.; Blaser, M.J. A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates Helicobacter pylori CagA Epithelial Cell Interactions. Plos Pathog. 2015, 11. [Google Scholar] [CrossRef] [Green Version]
- Glowinski, F.; Holland, C.; Thiede, B.; Jungblut, P.R.; Meyer, T.F. Analysis of T4SS-induced signaling by H. pylori using quantitative phosphoproteomics. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Sgouras, D.N.; Trang, T.T.; Yamaoka, Y. Pathogenesis of Helicobacter pylori Infection. Helicobacter 2015, 20 (Suppl. 1), 8–16. [Google Scholar] [CrossRef] [Green Version]
- Boquet, P.; Ricci, V. Intoxication strategy of Helicobacter pylori VacA toxin. Trends Microbiol. 2012, 20, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Leyton, D.L.; Rossiter, A.E.; Henderson, I.R. From self sufficiency to dependence: Mechanisms and factors important for autotransporter biogenesis. Nature Rev. Microbiol. 2012, 10, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Woo, H.; Park, M.; Rhee, K.J.; Moon, C.; Lee, D.; Seo, W.D.; Kim, J.B. Cyanidin 3-O-glucoside reduces Helicobacter pylori VacA-induced cell death of gastric KATO III cells through inhibition of the SecA pathway. Int. J. Med. Sci. 2014, 11, 742–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memon, A.A.; Hussein, N.R.; Miendje Deyi, V.Y.; Burette, A.; Atherton, J.C. Vacuolating cytotoxin genotypes are strong markers of gastric cancer and duodenal ulcer-associated Helicobacter pylori strains: A matched case-control study. J. Clin. Microbiol. 2014, 52, 2984–2989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitharwal, R.G.; Verma, V.; Dasgupta, S.; Dhar, S.K. Helicobacter pylori chromosomal DNA replication: Current status and future perspectives. FEBS Lett. 2011, 585, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, S.; Chung, B.H.; Lee, M.; Ha, T.H. Ring-shaped replicative helicase encircles double-stranded DNA during unwinding. Nucleic Acids Res. 2019, 47, 11344–11354. [Google Scholar] [CrossRef]
- Dewar, J.M.; Walter, J.C. Mechanisms of DNA replication termination. Nat. Rev. Mol. Cell Biol. 2017, 18, 507–516. [Google Scholar] [CrossRef]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of beta-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol--from the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef]
- Ghelardini, C.; Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A. Local anaesthetic activity of beta-caryophyllene. Farmaco 2001, 56, 387–389. [Google Scholar] [CrossRef]
- De Almeida Borges, V.R.; Ribeiro, A.F.; de Souza Anselmo, C.; Cabral, L.M.; de Sousa, V.P. Development of a high performance liquid chromatography method for quantification of isomers beta-caryophyllene and alpha-humulene in copaiba oleoresin using the Box-Behnken design. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 940, 35–41. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E. beta-Caryophyllene as a Potential Protective Agent Against Myocardial Injury: The Role of Toll-Like Receptors. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crevelin, E.J.; Caixeta, S.C.; Dias, H.J.; Groppo, M.; Cunha, W.R.; Martins, C.H.; Crotti, A.E. Antimicrobial Activity of the Essential Oil of Plectranthus neochilus against Cariogenic Bacteria. Evid. Based Complement. Alternat. Med. 2015, 2015, 102317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, E.; Bail, S.; Friedl, S.M.; Jirovetz, L.; Buchbauer, G.; Wanner, J.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Antimicrobial activities of single aroma compounds. Nat. Prod. Commun. 2010, 5, 1365–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturi, C.R.; Danielli, L.J.; Klein, F.; Apel, M.A.; Montanha, J.A.; Bordignon, S.A.; Roehe, P.M.; Fuentefria, A.M.; Henriques, A.T. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia. Pharm. Biol. 2015, 53, 682–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, M.; Stojkovic, D.; Glamoclija, J.; Ciric, A.; Markovic, T.; Smiljkovic, M.; Sokovic, M. Could essential oils of green and black pepper be used as food preservatives? J. Food Sci. Tech. Mys. 2015, 52, 6565–6573. [Google Scholar] [CrossRef] [Green Version]
- Heltzel, J.M.; Scouten Ponticelli, S.K.; Sanders, L.H.; Duzen, J.M.; Cody, V.; Pace, J.; Snell, E.H.; Sutton, M.D. Sliding clamp-DNA interactions are required for viability and contribute to DNA polymerase management in Escherichia coli. J. Mol. Biol. 2009, 387, 74–91. [Google Scholar] [CrossRef] [Green Version]
- Song, M.S.; Pham, P.T.; Olson, M.; Carter, J.R.; Franden, M.A.; Schaaper, R.M.; McHenry, C.S. The delta and delta’ subunits of the DNA polymerase III holoenzyme are essential for initiation complex formation and processive elongation. J. Biol. Chem. 2001, 276, 35165–35175. [Google Scholar] [CrossRef] [Green Version]
- De Souza Mendes, C.; de Souza Antunes, A.M. Pipeline of Known Chemical Classes of Antibiotics. Antibiotics (Basel) 2013, 2, 500–534. [Google Scholar] [CrossRef] [Green Version]
- Luan, G.; Drlica, K. Fluoroquinolone-Gyrase-DNA Cleaved Complexes. Methods Mol. Biol. 2018, 1703, 269–281. [Google Scholar] [CrossRef]
- Zhang, R.G.; Duan, G.C.; Fan, Q.T.; Chen, S.Y. Role of Helicobacter pylori infection in pathogenesis of gastric carcinoma. World J. Gastrointest. Pathophysiol. 2016, 7, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, T.; Loell, E.; Mueller, S.; Stoeckelhuber, M.; Stolte, M.; Haas, R.; Rieder, G. Helicobacter pylori cag-Pathogenicity island-dependent early immunological response triggers later precancerous gastric changes in Mongolian gerbils. PLoS ONE 2009, 4, e4754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillon, W.G.; Glomski, C.A. The Mongolian gerbil: Qualitative and quantitative aspects of the cellular blood picture. Lab. Anim. 1975, 9, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.A.; Glomski, C.A. The Mongolian gerbil (Meriones unguiculatus): Its histological and haematological response to methylcellulose. Lab. Anim. 1981, 15, 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambe, Y.; Tsujiuchi, H.; Honda, G.; Ikeshiro, Y.; Tanaka, S. Gastric cytoprotection of the non-steroidal anti-inflammatory sesquiterpene, beta-caryophyllene. Planta Med. 1996, 62, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, M.; Woo, H.; Tharmalingam, N.; Lee, G.; Rhee, K.J.; Eom, Y.B.; Han, S.I.; Seo, W.D.; Kim, J.B. Inhibitory effects of anthocyanins on secretion of Helicobacter pylori CagA and VacA toxins. Int. Med. Sci. 2012, 9, 838–842. [Google Scholar] [CrossRef] [Green Version]
- Garhart, C.A.; Redline, R.W.; Nedrud, J.G.; Czinn, S.J. Clearance of Helicobacter pylori Infection and Resolution of Postimmunization Gastritis in a Kinetic Study of Prophylactically Immunized Mice. Infect. Immun. 2002, 70, 3529–3538. [Google Scholar] [CrossRef] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, H.J.; Yang, J.Y.; Lee, M.H.; Kim, H.W.; Kwon, H.J.; Park, M.; Kim, S.-k.; Park, S.-Y.; Kim, S.-H.; Kim, J.-B. Inhibitory Effects of β-Caryophyllene on Helicobacter pylori Infection In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 1008. https://doi.org/10.3390/ijms21031008
Woo HJ, Yang JY, Lee MH, Kim HW, Kwon HJ, Park M, Kim S-k, Park S-Y, Kim S-H, Kim J-B. Inhibitory Effects of β-Caryophyllene on Helicobacter pylori Infection In Vitro and In Vivo. International Journal of Molecular Sciences. 2020; 21(3):1008. https://doi.org/10.3390/ijms21031008
Chicago/Turabian StyleWoo, Hyun Jun, Ji Yeong Yang, Min Ho Lee, Hyun Woo Kim, Hye Jin Kwon, Min Park, Sung-kyu Kim, So-Young Park, Sa-Hyun Kim, and Jong-Bae Kim. 2020. "Inhibitory Effects of β-Caryophyllene on Helicobacter pylori Infection In Vitro and In Vivo" International Journal of Molecular Sciences 21, no. 3: 1008. https://doi.org/10.3390/ijms21031008