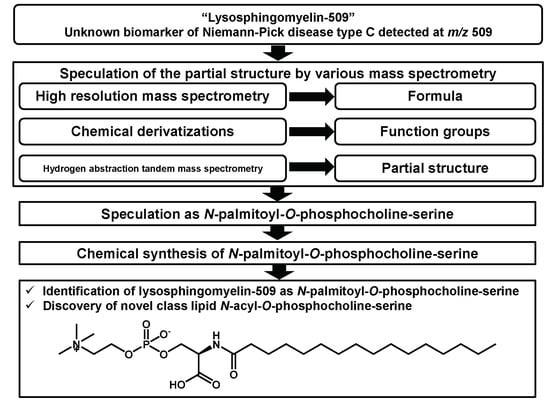
Structural Determination of Lysosphingomyelin-509 and Discovery of Novel Class Lipids from Patients with Niemann–Pick Disease Type C
Abstract
:1. Introduction
2. Result and Discussion
2.1. Structural Speculation of Lyso-SM-509
2.2. Identification of Lyso-SM-509
2.3. Analysis of Plasma/Serum Novel Class Lipids
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Structural Speculation of Lyso-SM-509
3.3. Chemical Derivatization for Lyso-SM-509 and SPC
3.4. Chemical Synthesis of N-Palmitoyl-O-Phosphocholine-Serine
3.5. Identification of Lyso-SM-509
3.6. Simultaneous Analysis of N-Palmitoyl-O-Phosphocholine-Serine and SPC in the Serum or Plasma
3.7. Targeted Lipidomics Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CHO | Chinese hamster ovary |
| Lyso-SM | Lysosphingomyelin |
| Lyso-SM-509 | Lysosphingomyelin-509 |
| NBD | 7-Nitro-2,1,3-benzoxadiazole |
| NPC | Niemann–Pick disease type C |
| SPC | Sphingosylphosphocholine |
| SRM | Selected reaction monitoring |
| AcOEt | Ethyl acetate |
| CDCl3 | Chloroform-d |
| CD3OD | Methanol-d4 |
| DCC | N, N’-Dicyclohexylcarbodiimide |
| DHB | 2, 5-Dihydroxbenzoic acid |
| Et3N | Triethylamin |
| HOBt | 1-Hydroxybenzotriazole |
| MeOH | Methanol |
| NMM | N-methylmorphorine |
| Ph | Phenyl |
| THF | Tetrahydrofuran |
References
- Vanier, M.T. Niemann-Pick type C disease. Orphanet J. Rare Dis. 2010, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Geberhiwot, T.; Moro, A.; Dardis, A.; Ramaswami, U.; Sirrs, S.; Marfa, M.P.; Vanier, M.T.; Walterfang, M.; Bolton, S.; Dawson, C.; et al. Consensus clinical management guidelines for Niemann-Pick disease type C. Orphanet J. Rare Dis. 2018, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Abi-Mosleh, L.; Wang, M.L.; Deisenhofer, J.; Goldstein, J.L.; Brown, M.S.; Infante, R.E. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 2009, 137, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Vanier, M.T. Biochemical studies in Niemann-Pick disease. I. Major sphingolipids of liver and spleen. Biochim. Biophys. Acta 1983, 750, 178–184. [Google Scholar] [CrossRef]
- Patterson, M.C.; Vecchio, D.; Prady, H.; Abel, L.; Wraith, J.E. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007, 6, 765–772. [Google Scholar] [CrossRef]
- Ory, D.S.; Ottinger, E.A.; Farhat, N.Y.; King, K.A.; Jiang, X.; Weissfeld, L.; Berry-Kravis, E.; Davidson, C.D.; Bianconi, S.; Keener, L.A.; et al. Intrathecal 2-hydroxypropyl-βncyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomized, open-label, phase 1-2 trial. Lancet 2017, 390, 1758–1768. [Google Scholar] [CrossRef]
- Porter, F.D.; Scherrer, D.E.; Lanier, M.H.; Langmade, S.J.; Molugu, V.; Gale, S.E.; Olzeski, D.; Sidhu, R.; Dietzen, D.J.; Fu, R.; et al. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med. 2010, 2, 56ra81. [Google Scholar] [CrossRef]
- Maekawa, M.; Misawa, Y.; Sotoura, A.; Yamaguchi, H.; Togawa, M.; Ohno, K.; Nittono, H.; Kakiyama, G.; Iida, T.; Hofmann, A.F.; et al. LC/ESI-MS/MS analysis of urinary 3β-sulfooxy-7β-N-acetylglucosaminyl-5-cholen-24-oic acid and its amides: New biomarkers for the detection of Niemann-Pick type C disease. Steroids 2013, 78, 967–972. [Google Scholar] [CrossRef]
- Mazzacuva, F.; Mills, P.; Mills, K.; Camuzeaux, S.; Gissen, P.; Elena-Raluca, N.; Wassif, C.; Vruchte, D.; Porter, F.D.; Maekawa, M.; et al. Identification of novel bile acids as biomarkers for the early diagnosis of Niemann-Pick C disease. FEBS Lett. 2016, 590, 1651–1662. [Google Scholar] [CrossRef] [Green Version]
- Welford, R.W.; Garzotti, M.; Lourenço, C.M.; Mengel, E.; Marquardt, T.; Reunart, J.; Amraoui, Y.; Kolb, S.A.; Morand, O.; Groenen, P. Plasma lysosphingomyelin demonstrates great potential as a diagnostic biomarker for Niemann-Pick disease type C in a retrospective study. PLoS ONE 2014, 9, e114669. [Google Scholar] [CrossRef]
- Giese, A.K.; Mascher, H.; Grittner, U.; Eichler, S.; Kramp, G.; Lukas, J.; Vruchte, D.; Eisa, N.; Cortina-Borja, M.; Porter, F.D.; et al. A novel, highly sensitive and specific biomarker for Niemann-Pick type C1 disease. Orphanet. J. Rare Dis. 2015, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.; Mondjinou, Y.; Qian, M.; Song, H.; Kumar, A.B.; Hong, X.; Hsu, F.F.; Dietzen, D.J.; Yanjanin, N.M.; Porter, F.D.; et al. N-acyl-O-phosphocholineserines: structure of a novel class lipid that are biomarkers for Niemann-Pick C1 disease. J. Lipid Res. 2019, 60, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Higaki, K.; Ninomiya, H.; Sugimoto, Y.; Suzuki, T.; Taniguchi, M.; Niwa, H.; Pentchev, P.G.; Vanier, M.T.; Ohno, K. Isolation of NPC1-deficient Chinese hamster ovary cell mutants by gene trap mutagenesis. J. Biochem. 2001, 129, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shimabukuro, Y.; Asakawa, D.; Yamauchi, S.; Sekiya, S.; Iwamoto, S.; Wada, M.; Tanaka, K. Structural analysis of phospholipid using hydrogen abstraction dissociation and oxygen attachment dissociation in tandem mass spectrometry. Anal. Chem. 2018, 90, 7230–7238. [Google Scholar] [CrossRef] [PubMed]
- Summer, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Vruchte, D.; Speak, A.O.; Wallom, K.L.; Eisa, N.A.; Smith, D.A.; Hendriksz, C.J.; Simmons, L.; Lachmann, R.H.; Cousins, A.; Hartung, R.; et al. Relative acidic compartment volume as a lysosomal storage disorder-associated biomarker. J. Clin. Investig. 2014, 124, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Leventis, P.A.; Grinstein, S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 2010, 39, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Li, S.; Smith, D.C.; Shaw, W.A.; Raetz, C.R.H. Identification of N-acylphosphatidylserine molecules in eukaryotic cells. Biochemistry 2007, 46, 14500–14513. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Tsuboi, K.; Okamoto, Y.; Hidaka, M.; Uyama, T.; Tsutsumi, T.; Tanaka, T.; Ueda, N.; Tokumura, A. Peripheral tissue levels and molecular species compositions of N-acyl-phosphatidylethanolamine and its metabolites in mice lacking N-acyl-phosphatidylethanolamine-specific phospholipase D. J. Biochem. 2017, 162, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Van Rooden, E.J.; Van Esbroeck, A.C.M.; Baggelaar, M.P.; Deng, H.; Florea, B.I.; Marques, A.R.A.; Ottenhoff, R.; Boot, R.G.; Overkleeft, H.S.; Aerts, J.M.F.G.; et al. Chemical proteomic analysis of serine hydrolase activity in Niemann-Pick type C mouse brain. Front. Neurosci. 2018, 12, 440. [Google Scholar]
- Wood, P.L. Accumulation of N-acylphosphatidylserines and N-acylserines in the frontal cortex in schizophrenia. Neurotransmitter (Houst) 2014, 1, e263. [Google Scholar] [PubMed]
- Arnold, R.S.; Cornell, R.B. Lipid regulation of CTP: phosphocholine cytidyltransferase: electrostatic, hydrophobic, and synergistic interactions of anionic phospholipids and diacylglycerol. Biochemistry 1996, 35, 9917–9924. [Google Scholar] [CrossRef] [PubMed]
- Huitema, K.; van den Dikkenberg, J.; Brouwers, J.F.; Holthuis, J.C. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004, 23, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, M.; Sayer, C.; Dannecker, R.; Schuler, W.; Sedrani, R. The macrolide everolimus forms an unusual metabolite in animals and humans: Identification of a phosphocholine ester. Drug Metab. Dispos. 2008, 36, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Cantone, J.L.; Wang, Y.; Leet, J.E.; Drexler, D.M.; Yeung, K.S.; Huang, X.S.; Eastman, K.J.; Parcella, K.E.; Mosure, K.W.; et al. Phosphocholine conjugation: An unexpected in vivo conjugation pathway associated with hepatitis C NS5B inhibitors featuring a bicycle[1.1.1]pentane. Drug Metab. Dispos. 2016, 44, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Deodato, F.; Boenzi, S.; Taurisano, R.; Semeraro, M.; Sacchetti, E.; Carrozzo, R.; Dionisi-Vici, G. The impact of biomarkers analysis in the diagnosis of Niemann-Pick C disease and acid sphingomyelinase deficiency. Clin. Chim. Acta 2018, 486, 387–394. [Google Scholar] [CrossRef]
- Voorink-Moret, M.; Goorden, S.M.I.; van Kuilenburg, A.B.P.; Wijburg, F.A.; Ghauharali-van der Vlugt, J.M.M.; Beers-Stet, F.S.; Zoetekouw, A.; Kulik, W.; Hollak, C.E.M.; Vaz, F.M. Rapid screening for lipid storage disorders using biochemical markers. Expert center data and review of the literature. Mol. Genet. Metab. 2018, 123, 76–84. [Google Scholar] [CrossRef]
- Arenas, F.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Intracellular cholesterol trafficking and impact in neurodegeneration. Front. Mol. Neurosci. 2017, 10, 382. [Google Scholar] [CrossRef]
- Pol, A.; Gross, S.P.; Parton, R.G. Biogenesis of the multifunctional lipid droplet: Lipids, proteins, and sites. J. Cell. Biol. 2014, 204, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Plemel, J.R.; Michaels, N.J.; Weishaupt, N.; Caprariello, A.V.; Keough, M.B.; Rogers, J.A.; Yukseloglu, A.; Lim, J.; Patel, V.V.; Rawji, K.S.; et al. Mechanisms of lysophosphatidylcholine-induced demyelination: A primary lipid disrupting myelinopathy. Glia 2018, 66, 327–347. [Google Scholar] [CrossRef]
- Jan, C.R.; Lu, Y.C.; Jiann, B.P.; Chang, H.T.; Wang, J.L.; Chen, W.C.; Huang, J.K. Novel effect of N-palmitoyl-L-serine phosphoric acid on cytosolic Ca2+ levels in human osteoblasts. Pharmacol. Toxicol. 2003, 93, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Pentchev, P.G.; Comly, M.E.; Kruth, H.S.; Vanier, M.T.; Wenger, D.A.; Patel, S.; Brady, R.O. A defect in cholesterol esterification in Niemann-Pick disease (type C) patients. Proc. Natl. Acad. Sci. USA 1985, 82, 8247–8251. [Google Scholar] [CrossRef] [PubMed]
- Carreau, J.P.; Dubacq, J.P. Adaptation of a macro-scale method to the micro-scale fatty acid methyl transesterification of biological lipid extracts. J. Chroromatogr. 1978, 151, 384–390. [Google Scholar] [CrossRef]
- Fritz, J.S.; Schenk, G.H. Acid-catalyzed acetylation of organic hydroxyl groups. Anal. Chem. 1959, 31, 1808–1812. [Google Scholar] [CrossRef]
- Koga, R.; Miyoshi, Y.; Negishi, E.; Kaneko, T.; Mita, M.; Lindner, W.; Hamase, K. Enantioselective two-dimensional high-performance liquid chromatographic determination of N-methyl-D-aspartic acid and its analogues in mammals and bivalves. J. Chromatogr. A 2012, 1269, 255–261. [Google Scholar] [CrossRef]
- Ishii, C.; Akita, T.; Mita, M.; Ide, T.; Hamase, K. Development of an online two-dimensional high-performance liquid chromatographic system in combination with tandem mass spectrometric detection for enantiomeric analysis of free amino acids in human physiological fluid. J. Chromatogr. A 2018, 1570, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Chung, H.M.; Park, J.S.; Choi, W.; Min, J.; Park, N.H.; Kim., K.H.; Jhon, G.J.; Han, S.Y. Synthesis of novel lysophosphatidylcholine analogues using serine as chiral template. J. Org. Chem. 2003, 68, 10162–10165. [Google Scholar] [CrossRef] [PubMed]
- Bartel, M.; Rattery, B.; Nuhn, P. Synthesis of enantiomerically pure, sn-1 modified sn-2-deoxy-2-amido-glycero-3-phospholipids. Chem. Phys. Lipids 2000, 107, 121–129. [Google Scholar] [CrossRef]
- Bioanalytical Method Validation: Guidance for Industry. Available online: http://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 27 June 2018).








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maekawa, M.; Jinnoh, I.; Matsumoto, Y.; Narita, A.; Mashima, R.; Takahashi, H.; Iwahori, A.; Saigusa, D.; Fujii, K.; Abe, A.; et al. Structural Determination of Lysosphingomyelin-509 and Discovery of Novel Class Lipids from Patients with Niemann–Pick Disease Type C. Int. J. Mol. Sci. 2019, 20, 5018. https://doi.org/10.3390/ijms20205018
Maekawa M, Jinnoh I, Matsumoto Y, Narita A, Mashima R, Takahashi H, Iwahori A, Saigusa D, Fujii K, Abe A, et al. Structural Determination of Lysosphingomyelin-509 and Discovery of Novel Class Lipids from Patients with Niemann–Pick Disease Type C. International Journal of Molecular Sciences. 2019; 20(20):5018. https://doi.org/10.3390/ijms20205018
Chicago/Turabian StyleMaekawa, Masamitsu, Isamu Jinnoh, Yotaro Matsumoto, Aya Narita, Ryuichi Mashima, Hidenori Takahashi, Anna Iwahori, Daisuke Saigusa, Kumiko Fujii, Ai Abe, and et al. 2019. "Structural Determination of Lysosphingomyelin-509 and Discovery of Novel Class Lipids from Patients with Niemann–Pick Disease Type C" International Journal of Molecular Sciences 20, no. 20: 5018. https://doi.org/10.3390/ijms20205018