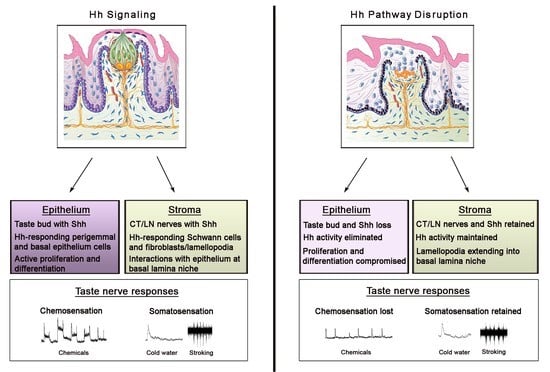
Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation
Abstract
:1. Introduction
2. Development of the Taste Papilla Organ, the Role of Innervation, and Hh Signaling
3. Shh and Shh Signaling Locations Postnatally and in the Adult Tongue and Taste Organs
4. Epithelial Hh Signaling is Essential for Homeostasis and Reconstitution of TB and Chemosensation; Innervation Alone is not Sufficient for TB Maintenance
4.1. Hh Pathway Inhibition with Sonidegib: Taste Organ and Sensory Functional Effects
4.2. Recovery from Hh Pathway Disruption
4.3. Nerves are not Sufficient for TB Maintenance or Restoration after Hh Signaling Disruption
5. Lingual Innervation and Hh Signaling: TB Maintenance and Regeneration in Nerve Cut Studies and BDNF Requirement
5.1. Nerve-Dependence and Degeneration/Regeneration of Taste Buds
5.2. Innervation and Shh Signaling
5.3. Removing the FP Organ and Hh-Responding TB Progenitors
6. Sources of the Shh Ligand in TB Cells and in Nerves; Potential Distinctive Roles
6.1. Sources of the Sonic Hedgehog Ligand and Signaling in TB Cells and in Ganglion Soma and Nerves
6.2. Distinctive Roles for Hh Signaling in the Epithelium Versus Stroma
7. Taste Organ Niches and the Basal Lamina in Hh Signaling
8. Chemosensation, Somatosensation, Hh Signaling, and Disrupted Taste Perception
8.1. Lingual Taste, Touch, and Cold Sensation after TB Alterations by HPI Drugs: Animal Studies
8.2. Lingual Taste Sensation after Nerve and Taste Bud Disruptions: Patient/Clinical Studies and Hh Signaling
9. Concluding Remarks: Hh Signaling in Taste Papillae
9.1. Shh and Signaling in Gustatory Papillae, the TBs and in Nerves: Necessary and Sufficient
9.2. Roles for the Basal Lamina
9.3. Proposed Roles for Neural Shh in Peripheral Taste Organ Function
9.4. Summary and Future Directions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FP | Fungiform papilla |
| CV | Circumvallate papilla |
| TB | Taste bud |
| CT | Chorda tympani |
| GL | Glossopharyngeal nerve |
| LN | Lingual nerve |
| Shh | Sonic hedgehog |
| Hh | Hedgehog |
| GG | Geniculate ganglion |
| TG | Trigeminal ganglion |
References
- Liu, H.X.; Ermilov, A.; Grachtchouk, M.; Li, L.; Gumucio, D.L.; Dlugosz, A.A.; Mistretta, C.M. Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev. Biol. 2013, 382, 82–97. [Google Scholar] [CrossRef] [Green Version]
- Castillo, D.; Seidel, K.; Salcedo, E.; Ahn, C.; de Sauvage, F.J.; Klein, O.D.; Barlow, L.A. Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium. Development 2014, 141, 2993–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.-J.; Mann, R.K.; Nguyen, A.; Bi, T.; Silverstein, M.; Tang, J.Y.; Chen, X.; Beachy, P.A. Neuronal delivery of Hedgehog directs spatial patterning of taste organ regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E200–E209. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Azofeifa, D.; Losacco, J.T.; Salcedo, E.; Golden, E.J.; Finger, T.E.; Barlow, L.A. Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance. Development 2017, 144, 3054–3065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.; Ermilov, A.N.; Allen, B.L.; Bradley, R.M.; Dlugosz, A.A.; Mistretta, C.M. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J. Neurophysiol. 2015, 113, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Ermilov, A.N.; Grachtchouk, M.; Dlugosz, A.A.; Allen, B.L.; Bradley, R.M.; Mistretta, C.M. Recovery of taste organs and sensory function after severe loss from Hedgehog/Smoothened inhibition with cancer drug sonidegib. Proc. Natl. Acad. Sci. USA 2017, 114, E10369–E10378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.; Yokota, Y.; Li, L.; Bradley, R.M.; Mistretta, C.M. Species generalization and differences in Hedgehog pathway regulation of fungiform and circumvallate papilla taste function and somatosensation demonstrated with sonidegib. Sci. Rep. 2018, 8, 16150. [Google Scholar] [CrossRef]
- Yang, H.; Cong, W.N.; Yoon, J.S.; Egan, J.M. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 2015, 4, 245–252. [Google Scholar] [CrossRef]
- Ermilov, A.N.; Kumari, A.; Li, L.; Joiner, A.M.; Grachtchouk, M.A.; Allen, B.L.; Dlugosz, A.A.; Mistretta, C.M. Maintenance of taste organs is strictly dependent on epithelial Hedgehog/GLI signaling. PLoS Genet. 2016, 12, e1006442. [Google Scholar] [CrossRef] [PubMed]
- Nybakken, K.; Perrimon, N. Heparan sulfate proteoglycan modulation of developmental signaling in Drosophila. Biochim. Biophys. Acta 2002, 1573, 280–291. [Google Scholar] [CrossRef]
- Barlow, L.A. Progress and renewal in gustation: New insights into taste bud development. Development 2015, 142, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Barlow, L.A.; Klein, O.D. Developing and regenerating a sense of taste. Curr. Top. Dev. Biol. 2015, 111, 401–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistretta, C.M.; Kumari, A. Tongue and Taste Organ Biology and Function:Homeostasis Maintained by Hedgehog Signaling. Annu. Rev. Physiol. 2017, 79, 335–356. [Google Scholar] [CrossRef]
- Miura, H.; Kusakabe, Y.; Harada, S. Cell lineage and differentiation in taste buds. Arch. Histol. Cytol. 2006, 69, 209–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbiene, J.P.; Mistretta, C.M. Initial innervation of embryonic rat tongue and developing taste papillae: Nerves follow distinctive and spatially restricted pathways. Acta Anat (Basel) 1997, 160, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Mbiene, J.P.; Maccallum, D.K.; Mistretta, C.M. Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J. Comp. Neurol. 1997, 377, 324–340. [Google Scholar] [CrossRef]
- Mistretta, C.M.; Liu, H.X. Development of fungiform papillae: Patterned lingual gustatory organs. Arch. Histol. Cytol. 2006, 69, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistretta, C.M.; Liu, H.X.; Gaffield, W.; MacCallum, D.K. Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: Fungiform papillae double in number and form in novel locations in dorsal lingual epithelium. Dev. Biol. 2003, 254, 1–18. [Google Scholar] [CrossRef]
- Hall, J.M.; Hooper, J.E.; Finger, T.E. Expression of sonic hedgehog, patched, and Gli1 in developing taste papillae of the mouse. J. Comp. Neurol. 1999, 406, 143–155. [Google Scholar] [CrossRef]
- Liu, H.X.; Maccallum, D.K.; Edwards, C.; Gaffield, W.; Mistretta, C.M. Sonic hedgehog exerts distinct, stage-specific effects on tongue and taste papilla development. Dev. Biol. 2004, 276, 280–300. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.M.; Bell, M.L.; Finger, T.E. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev. Biol. 2003, 255, 263–277. [Google Scholar] [CrossRef] [Green Version]
- El Shahawy, M.; Reibring, C.G.; Neben, C.L.; Hallberg, K.; Marangoni, P.; Harfe, B.D.; Klein, O.D.; Linde, A.; Gritli-Linde, A. Cell fate specification in the lingual epithelium is controlled by antagonistic activities of Sonic hedgehog and retinoic acid. PLoS Genet. 2017, 13, e1006914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, H.X.; Mistretta, C.M. Bone morphogenetic proteins and noggin: Inhibiting and inducing fungiform taste papilla development. Dev. Biol. 2006, 297, 198–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.X.; Henson, B.S.; Zhou, Y.; D’Silva, N.J.; Mistretta, C.M. Fungiform papilla pattern: EGF regulates inter-papilla lingual epithelium and decreases papilla number by means of PI3K/Akt, MEK/ERK, and p38 MAPK signaling. Dev. Dyn. 2008, 237, 2378–2393. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Grosse, A.M.; Walton, K.D.; Saims, D.A.; Gumucio, D.L.; Mistretta, C.M. WNT5a in tongue and fungiform Papilla development. Ann. N. Y. Acad. Sci. 2009, 1170, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Grosse, A.S.; Iwatsuki, K.; Mishina, Y.; Gumucio, D.L.; Mistretta, C.M. Separate and distinctive roles for Wnt5a in tongue, lingual tissue and taste papilla development. Dev. Biol. 2012, 361, 39–56. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Liu, H.X.; Gronder, A.; Singer, M.A.; Lane, T.F.; Grosschedl, R.; Mistretta, C.M.; Margolskee, R.F. Wnt signaling interacts with Shh to regulate taste papilla development. Proc. Natl. Acad. Sci. USA 2007, 104, 2253–2258. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Thirumangalathu, S.; Gallant, N.M.; Yang, S.H.; Stoick-Cooper, C.L.; Reddy, S.T.; Andl, T.; Taketo, M.M.; Dlugosz, A.A.; Moon, R.T.; et al. Wnt-β-catenin signaling initiates taste papilla development. Nat. Genet. 2007, 39, 106. [Google Scholar] [CrossRef]
- Petersen, C.I.; Jheon, A.H.; Mostowfi, P.; Charles, C.; Ching, S.; Thirumangalathu, S.; Barlow, L.A.; Klein, O.D. FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field Size. PLoS Genet. 2011, 7, e1002098. [Google Scholar] [CrossRef]
- Krimm, R.F.; Miller, K.K.; Kitzman, P.H.; Davis, B.M.; Albers, K.M. Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Dev. Biol. 2001, 232, 508–521. [Google Scholar] [CrossRef]
- Lopez, G.F.; Krimm, R.F. Epithelial overexpression of BDNF and NT4 produces distinct gustatory axon morphologies that disrupt initial targeting. Dev. Biol. 2006, 292, 457–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirumangalathu, S.; Harlow, D.E.; Driskell, A.L.; Krimm, R.F.; Barlow, L.A. Fate mapping of mammalian embryonic taste bud progenitors. Development 2009, 136, 1519–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, H.; Kato, H.; Kusakabe, Y.; Tagami, M.; Miura-Ohnuma, J.; Ookura, T.; Shindo, Y.; Ninomiya, Y.; Hino, A. Shh signaling and regulatory gene expression in mouse taste buds. Chem. Senses 2005, 30 (Suppl. 1), i50–i51. [Google Scholar] [CrossRef]
- Miura, H.; Kusakabe, Y.; Sugiyama, C.; Kawamatsu, M.; Ninomiya, Y.; Motoyama, J.; Hino, A. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech. Dev. 2001, 106, 143–145. [Google Scholar] [CrossRef]
- Okubo, T.; Clark, C.; Hogan, B.L. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem. Cells 2009, 27, 442–450. [Google Scholar] [CrossRef]
- Miura, H.; Scott, J.K.; Harada, S.; Barlow, L.A. Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Dev. Dyn. 2014, 243, 1286–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beidler, L.M.; Smallman, R.L. Renewal of cells within taste buds. J. Cell Biol. 1965, 27, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Therond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef]
- Qin, Y.; Sukumaran, S.K.; Jyotaki, M.; Redding, K.; Jiang, P.; Margolskee, R.F. Gli3 is a negative regulator of Tas1r3-expressing taste cells. PLoS Genet. 2018, 14, e1007058. [Google Scholar] [CrossRef]
- Guagliardo, N.A.; Hill, D.L. Fungiform taste bud degeneration in C57BL/6J mice following chorda-lingual nerve transection. J. Comp. Neurol. 2007, 504, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.; Lawton, A.; Riddle, D.R.; Wu, L.H. Morphometric and immunocytochemical assessment of fungiform taste buds after interruption of the chorda-lingual nerve. Microsc. Res. Tech. 1993, 26, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Segerstad, C.H.A.; Hellekant, G.; Farbman, A.I. Changes in number and morphology of fungiform taste buds in rat after transection of the chorda tympani or chordalingual nerve. Chem. Senses 1989, 14, 335–348. [Google Scholar] [CrossRef]
- Guth, L. Taste buds on the cat’s circumvallate papilla after reinnervation by glossopharyngeal, vagus, and hypoglossal nerves. Anat. Rec. 1958, 130, 25–37. [Google Scholar] [CrossRef]
- Zalewski, A.A. Regeneration of taste buds after reinnervation of a denervated tongue papilla by a normally nongustatory nerve. J. Comp. Neurol. 1981, 200, 309–314. [Google Scholar] [CrossRef]
- von Vintschgau, M.; Honigschmied, J. Nervus glossopharyngeus und Schmeckbecher. Pflügers Arch. 1876, 14, 443–448. [Google Scholar] [CrossRef]
- Smith, D.V.; Klevitsky, R.; Akeson, R.A.; Shipley, M.T. Expression of the neural cell adhesion molecule (NCAM) and polysialic acid during taste bud degeneration and regeneration. J. Comp. Neurol. 1994, 347, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Suzuki, Y.; Obara, N.; Breipohl, W. Expression of the neural cell adhesion molecule in mouse taste buds after denervation. J. Electron. Microsc. 1999, 48, 39–45. [Google Scholar] [CrossRef]
- Yee, C.; Bartel, D.L.; Finger, T.E. Effects of glossopharyngeal nerve section on the expression of neurotrophins and their receptors in lingual taste buds of adult mice. J. Comp. Neurol 2005, 490, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Seta, Y.; Toyono, T.; Takeda, S.; Toyoshima, K. Expression of Mash1 in basal cells of rat circumvallate taste buds is dependent upon gustatory innervation. FEBS Lett. 1999, 444, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Cheal, M.; Oakley, B. Regeneration of fungiform taste buds: Temporal and spatial characteristics. J. Comp. Neurol. 1977, 172, 609–626. [Google Scholar] [CrossRef]
- Sollars, S.I.; Bernstein, I.L. Neonatal chorda tympani transection permanently disrupts fungiform taste bud and papilla structure in the rat. Physiol. Behav. 2000, 69, 439–444. [Google Scholar]
- Sollars, S.I.; Smith, P.C.; Hill, D.L. Time course of morphological alterations of fungiform papillae and taste buds following chorda tympani transection in neonatal rats. J. Neurobiol. 2002, 51, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Yang, J.M.; Huang, Y.B.; Ren, D.D.; Chi, F.L. Shrinkage of ipsilateral taste buds and hyperplasia of contralateral taste buds following chorda tympani nerve transection. Neural Regen Res. 2015, 10, 989–995. [Google Scholar] [CrossRef]
- Sollars, S.I. Chorda tympani nerve transection at different developmental ages produces differential effects on taste bud volume and papillae morphology in the rat. J. Neurobiol. 2005, 64, 310–320. [Google Scholar] [CrossRef]
- Cain, P.; Frank, M.E.; Barry, M.A. Recovery of chorda tympani nerve function following injury. Exp. Neurol. 1996, 141, 337–346. [Google Scholar] [CrossRef]
- Whitehead, M.C.; Ganchrow, J.R.; Ganchrow, D.; Yao, B. Neural cell adhesion molecule, neuron-specific enolase and calcitonin gene-related peptide immunoreactivity in hamster taste buds after chorda tympani/lingual nerve denervation. Neuroscience 1998, 83, 843–856. [Google Scholar] [CrossRef]
- Meng, L.; Ohman-Gault, L.; Ma, L.; Krimm, R.F. Taste Bud-Derived BDNF Is Required to Maintain Normal Amounts of Innervation to Adult Taste Buds. eNeuro 2015, 2. [Google Scholar] [CrossRef]
- Meng, L.; Huang, T.; Sun, C.; Hill, D.L.; Krimm, R. BDNF is required for taste axon regeneration following unilateral chorda tympani nerve section. Exp. Neurol. 2017, 293, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Rios-Pilier, J.; Krimm, R. Taste bud-derived BDNF maintains innervation of a subset of TrkB-expressing gustatory nerve fibers. Mol. Cell. Neurosci. 2017, 82, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Dai, R.L.; Zhu, S.Y.; Xia, Y.P.; Mao, L.; Mei, Y.W.; Yao, Y.F.; Xue, Y.M.; Hu, B. Sonic hedgehog protects cortical neurons against oxidative stress. Neurochem. Res. 2011, 36, 67–75. [Google Scholar] [CrossRef]
- He, W.; Cui, L.; Zhang, C.; Zhang, X.; He, J.; Xie, Y. Sonic Hedgehog Promotes Neurite Outgrowth of Primary Cortical Neurons Through Up-Regulating BDNF Expression. Neurochem. Res. 2016, 41, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, J.L.; Wan, X.X.; Song, Z.J.; Miao, S.; Zhao, Y.; Wang, X.L.; Liu, Y.P. Sonic hedgehog signaling in spinal cord contributes to morphine-induced hyperalgesia and tolerance through upregulating brain-derived neurotrophic factor expression. J. Pain Res. 2018, 11, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, C.W.; Angeloni, N.; Harrington, D.; Stupp, S.; Podlasek, C.A. Sonic Hedgehog regulates brain-derived neurotrophic factor in normal and regenerating cavernous nerves. J. Sex. Med. 2013, 10, 730–737. [Google Scholar] [CrossRef]
- Radzikinas, K.; Aven, L.; Jiang, Z.; Tran, T.; Paez-Cortez, J.; Boppidi, K.; Lu, J.; Fine, A.; Ai, X. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J. Neurosci. 2011, 31, 15407–15415. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More Insight into BDNF against Neurodegeneration: Anti-Apoptosis, Anti-Oxidation, and Suppression of Autophagy. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Wu, C.L.; Chen, S.D.; Hwang, C.S.; Yang, D.I. Sonic hedgehog mediates BDNF-induced neuroprotection against mitochondrial inhibitor 3-nitropropionic acid. Biochem. Biophys. Res. Commun. 2009, 385, 112–117. [Google Scholar] [CrossRef]
- Wu, C.L.; Chen, S.D.; Yin, J.H.; Hwang, C.S.; Yang, D.I. Erythropoietin and sonic hedgehog mediate the neuroprotective effects of brain-derived neurotrophic factor against mitochondrial inhibition. Neurobiol. Dis. 2010, 40, 146–154. [Google Scholar] [CrossRef]
- Miura, H.; Kato, H.; Kusakabe, Y.; Tagami, M.; Miura-Ohnuma, J.; Ninomiya, Y.; Hino, A. A strong nerve dependence of sonic hedgehog expression in basal cells in mouse taste bud and an autonomous transcriptional control of genes in differentiated taste cells. Chem. Senses 2004, 29, 823–831. [Google Scholar] [CrossRef]
- Kumari, A.; Allen, B.L.; Bradley, R.M.; Dlugosz, A.A.; Mistretta, C.M. Role of innervation in HH signaling in the adult mouse fungiform taste papilla. In Proceedings of the Society for Neuroscience, San Diego, CA, USA, 12–16 November 2016. [Google Scholar]
- Mistretta, C.M.; Kumari, A.; Li, L.; Allen, B.L.; Bradley, R.M. Nerves and Sonic Hedgehog Signaling Interactions in Fungiform Papilla Taste Organ Homeostasis. In Proceedings of the Association for Chemoreception Sciences, Bonita Springs, FL, USA, 17–20 April 2018. [Google Scholar]
- Brownell, I.; Guevara, E.; Bai, C.B.; Loomis, C.A.; Joyner, A.L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 2011, 8, 552–565. [Google Scholar] [CrossRef]
- Xiao, Y.; Thoresen, D.T.; Williams, J.S.; Wang, C.; Perna, J.; Petrova, R.; Brownell, I. Neural Hedgehog signaling maintains stem cell renewal in the sensory touch dome epithelium. Proc. Natl. Acad. Sci. USA 2015, 112, 7195–7200. [Google Scholar] [CrossRef] [Green Version]
- Peterson, S.C.; Eberl, M.; Vagnozzi, A.N.; Belkadi, A.; Veniaminova, N.A.; Verhaegen, M.E.; Bichakjian, C.K.; Ward, N.L.; Dlugosz, A.A.; Wong, S.Y. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 2015, 16, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Hellekant, G.; Kasahara, Y.; Farbman, A.I.; Harada, S.; Segerstad, C.H.A. Regeneration ability of fungiform papillae and taste-buds in rats. Chem. Senses 1987, 12, 459–465. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 2014, 14, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Martinez, C.A.; Koester, J.; Wickstrom, S.A. Signaling in the stem cell niche: Regulating cell fate, function and plasticity. Development 2018, 145. [Google Scholar] [CrossRef]
- Morrison, S.J.; Spradling, A.C. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar] [PubMed]
- Voog, J.; Jones, D.L. Stem cells and the niche: A dynamic duo. Cell Stem Cell 2010, 6, 103–115. [Google Scholar] [CrossRef]
- Kelley, L.C.; Lohmer, L.L.; Hagedorn, E.J.; Sherwood, D.R. Traversing the basement membrane in vivo: A diversity of strategies. J. Cell Biol. 2014, 204, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Arends, F.; Lieleg, O. Biophysical Properties of the Basal Lamina: A Highly Selective Extracellular Matrix, Composition and Function of the Extracellular Matrix in the Human Body. In Composition and Function of the Extracellular Matrix in the Human Body; Travascio, F., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Allen, B.L.; Filla, M.S.; Rapraeger, A.C. Role of heparan sulfate as a tissue-specific regulator of FGF-4 and FGF receptor recognition. J. Cell Biol. 2001, 155, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Loper, H.B.; La Sala, M.; Dotson, C.; Steinle, N. Taste perception, associated hormonal modulation, and nutrient intake. Nutr. Rev. 2015, 73, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Fife, K.; Herd, R.; Lalondrelle, S.; Plummer, R.; Strong, A.; Jones, S.; Lear, J.T. Managing adverse events associated with vismodegib in the treatment of basal cell carcinoma. Future Oncol. 2017, 13, 175–184. [Google Scholar] [CrossRef]
- Jacobsen, A.A.; Aldahan, A.S.; Hughes, O.B.; Shah, V.V.; Strasswimmer, J. Hedgehog pathway inhibitor therapy for locally advanced and metastatic basal cell carcinoma: A systematic review and pooled analysis of interventional studies. JAMA Dermatol. 2016, 152, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Mackay-Wiggan, J.M.; Aszterbaum, M.; Yauch, R.L.; Lindgren, J.; Chang, K.; Coppola, C.; Chanana, A.M.; Marji, J.; Bickers, D.R.; et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N. Engl. J. Med. 2012, 366, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Shah, A.A.; Mistretta, C.M.; Bradley, R.M.; Pierchala, B.A. Biphasic functions for the GDNF-Ret signaling pathway in chemosensory neuron development and diversification. Proc. Natl. Acad. Sci. USA 2018, 115, E516–E525. [Google Scholar] [CrossRef] [PubMed]
- Snyder, D.J.; Bartoshuk, L.M. Oral sensory nerve damage: Causes and consequences. Rev. Endocr. Metab. Disord. 2016, 17, 149–158. [Google Scholar] [CrossRef]
- Kushnerev, E.; Yates, J.M. Evidence-based outcomes following inferior alveolar and lingual nerve injury and repair: A systematic review. J. Oral. Rehabil. 2015, 42, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.; Ahn, J.; Cho, Y.S. Taste Changes in Patients with Middle Ear Surgery by Intraoperative Manipulation of Chorda Tympani Nerve. Otol. Neurotol. 2018, 39, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Soldatova, L.; Doty, R.L. Post-tonsillectomy taste dysfunction: Myth or reality? World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 77–83. [Google Scholar] [CrossRef]
- Ziylan, F.; Smeeing, D.P.J.; Bezdjian, A.; Stegeman, I.; Thomeer, H. Feasibility of preservation of chorda tympani nerve during noninflammatory ear surgery: A systematic review. Laryngoscope 2018, 128, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.R.; Chen, N.; Phillips, C.L. Chemosensory and somatosensory regeneration after lingual nerve repair in humans. J. Oral. Maxillofac. Surg. 1997, 55, 2–13; discussion 13–14. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Catalanotto, F.; Hoffman, H.; Logan, H.; Snyder, D.J. Taste damage (otitis media, tonsillectomy and head and neck cancer), oral sensations and BMI. Physiol. Behav. 2012, 107, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Maeda, E.; Katsura, H.; Nin, T.; Sakaguchi-Fukunaga, A.; Mishiro, Y.; Sakagami, M. Change of somatosensory function of the tongue caused by chorda tympani nerve disorder after stapes surgery. Laryngoscope 2018, 128, 701–706. [Google Scholar] [CrossRef]
- Sakagami, M. Taste Disturbance and Its Recovery after Middle Ear Surgery. Chem. Senses 2005, 30, i220–i221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, A.; Seeberger, R.; Bittner, M.; Rolke, R.; Welte-Jzyk, C.; Daublander, M. Profiling intraoral neuropathic disturbances following lingual nerve injury and in burning mouth syndrome. BMC Oral Health 2017, 17, 68. [Google Scholar] [CrossRef]
- Seo, K.; Inada, Y.; Terumitsu, M.; Nakamura, T.; Shigeno, K.; Tanaka, Y.; Tsurumaki, T.; Kurata, S.; Matsuzawa, H. Protracted delay in taste sensation recovery after surgical lingual nerve repair: A case report. J. Med. Case Rep. 2013, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Teker, A.M.; Gedikli, O.; Altun, H.; Korkut, A.Y.; Ahishali, B. Chorda tympani nerve analysis with electron microscopy in chronic suppurative otitis media. Acta Otolaryngol. 2010, 130, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Mandel, L. Hyposalivation after undergoing stapedectomy. J. Am. Dent. Assoc. 2012, 143, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Mese, H.; Matsuo, R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 2007, 34, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Henkin, R.I.; Knoppel, A.B.; Abdelmeguid, M.; Stateman, W.A.; Hosein, S. Sonic hedgehog is present in parotid saliva and is decreased in patients with taste dysfunction. J. Oral Pathol. Med. 2017, 46, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Henkin, R.I.; Hosein, S.; Stateman, W.A.; Knoppel, A.B. Sonic Hedgehog in Nasal Mucus is a Biomarker for Smell Loss in patients with Hyposmia. Cell Mol. Med. 2016, 2, 2. [Google Scholar] [CrossRef]
- Saito, T.; Ito, T.; Kato, Y.; Yamada, T.; Manabe, Y.; Narita, N. Observation of regenerated fungiform taste buds after severing the chorda tympani nerve using confocal laser scanning microscopy in vivo. Otol. Neurotol. 2014, 35, e110–e116. [Google Scholar] [CrossRef]
- Saito, T.; Ito, T.; Ito, Y.; Manabe, Y. Long-term Follow-up Results of Regeneration Process of Fungiform Taste Buds After Severing the Chorda Tympani Nerve During Middle Ear Surgery. Ann. Otol. Rhinol. Laryngol. 2016, 125, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Narita, N.; Yamada, T.; Ogi, K.; Kanno, M.; Manabe, Y.; Ito, T. Length of nerve gap defects correlates with incidence of nerve regeneration but not with recovery of taste function in patients with severed chorda tympani nerve. Otol. Neurotol. 2011, 32, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Shibamori, Y.; Yamamoto, T.; Saito, T.; Manabe, Y.; Ohtsubo, T.; Yamagishi, T.; Saito, H. Morphological and Functional Study of Regenerated Chorda Tympani Nerves in Humans. Ann. Otol. Rhinol. Laryngol. 2000, 109, 703–709. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Donnelly, J.M.; Engevik, A.C.; Xiao, C.; Yang, L.; Kenny, S.; Varro, A.; Hollande, F.; Samuelson, L.C.; Zavros, Y. Gastric sonic hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology 2012, 142, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ohazama, A.; Maeda, T.; Seo, K. The Sonic Hedgehog signaling pathway regulates inferior alveolar nerve regeneration. Neurosci. Lett. 2018, 671, 114–119. [Google Scholar] [CrossRef]
- Feng, P.; Huang, L.; Wang, H. Taste bud homeostasis in health, disease, and aging. Chem. Senses 2014, 39, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Belgacem, Y.H.; Hamilton, A.M.; Shim, S.; Spencer, K.A.; Borodinsky, L.N. The Many Hats of Sonic Hedgehog Signaling in Nervous System Development and Disease. J. Dev. Biol. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Beidler, L.M. Innervation of Rat Fungiform Papillae, Olfaction and Taste III ed.; Pfaffmann, C., Ed.; Rockefeller University Press: New York, NY, USA, 1969. [Google Scholar]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mistretta, C.M.; Kumari, A. Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation. Int. J. Mol. Sci. 2019, 20, 1341. https://doi.org/10.3390/ijms20061341
Mistretta CM, Kumari A. Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation. International Journal of Molecular Sciences. 2019; 20(6):1341. https://doi.org/10.3390/ijms20061341
Chicago/Turabian StyleMistretta, Charlotte M., and Archana Kumari. 2019. "Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation" International Journal of Molecular Sciences 20, no. 6: 1341. https://doi.org/10.3390/ijms20061341