Chemo-Enzymatic Synthesis of Renewable Sterically-Hindered Phenolic Antioxidants with Tunable Polarity from Lignocellulose and Vegetal Oil Components
Abstract
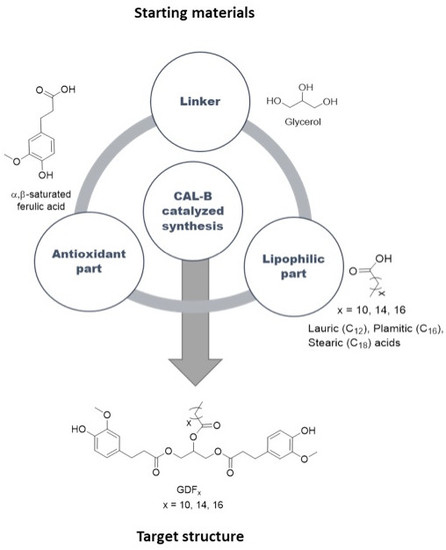
:1. Introduction
2. Experimental
2.1. Materials
2.2. Analytical Methods
2.3. Synthesis of Benzylated Ethyl Ferulate
2.4. Lipase-Catalyzed Transesterification of Benzylated Ethyl Ferulate into Glycerol Dibenzyl Ferulate (GDFoBn)
2.5. Lipophilization: Synthesis of GDFx
2.6. Calculation of Solubility Parameters
- Fdi: Dispersion contribution of the molar attraction constant [(J1/2 cm−3/2)/mol−1]
- Fpi: Polar contribution of the molar attraction constant [(J1/2 cm−3/2)/mol−1]
- Ehi: Hydrogen-bonding energy contribution of the molar attraction constant (J/mol)
- V: Molar volume contribution of the chemical group involved (cm3/mol).
2.7. Analysis of the Radical Scavenging Power of Antioxidants
3. Results and Discussions
3.1. Design of Lipophilic Antioxidants: Predictive Approaches
3.2. Synthesis of the Targets (GDFx)
- (I)
- Stoichiometric one pot-one step enzymatic strategy,
- (II)
- One pot-two step strategy, and
- (III)
- Chemo-enzymatic strategy.
3.3. Stochiometric One Pot-One Step Enzymatic Strategy (Pathway I, Figure 7)
3.4. One Pot-Two Step Strategy
3.5. Chemo-Enzymatic Strategy
3.6. Analysis of the Antiradical Activity of Lipophilic Bisphenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zweifel, H. Stabilization of Polymeric Materials; Springer: New York, NY, USA, 1998. [Google Scholar]
- Zweifel, H. Polymer Durability: Degradation, Stabilization, and Lifetime Prediction; ACS Publishing: New York, NY, USA, 1996. [Google Scholar]
- Scott, G. Polymer Degradation and Stabilisation; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Sohma, J. Mechano-radical formation in polypropylene by an extruder action and its after-effects. Colloid Polym. Sci. 1992, 270, 1060–1065. [Google Scholar] [CrossRef]
- Verdu, J. Oxydative Ageing of Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bolland, J.L.; Gee, G. Kinetic studies in the chemistry of rubber and related materials. III. Thermochemistry and mechanisms of olefin oxidation. Trans. Faraday Soc. 1946, 42, 244–252. [Google Scholar] [CrossRef]
- Pritchard, G. Plastics Additives: An A-Z Reference; Polymer Science and Technology Series; Springer: Haarlem, The Netherlands, 2012. [Google Scholar]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; Taylor & Francis: Abingdon, UK, 1989. [Google Scholar]
- Pospíšil, J. Chemical and photochemical behaviour of phenolic antioxidants in polymer stabilization: A state of the art report, part II. Polym. Degrad. Stab. 1993, 39, 103–115. [Google Scholar] [CrossRef]
- Brewer, M. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Dimitrios, B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Gaspar, A.; Martins, M.; Silva, P.; Garrido, E.M.; Garrido, J.; Firuzi, O.; Miri, R.; Saso, L.; Borges, F.J. Dietary phenolic acids and derivatives. Evaluation of the antioxidant activity of sinapic acid and its alkyl esters. Agric. Food Chem. 2010, 58, 11273–11280. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H.J. Antioxidant properties of ferulic acid and its related compounds. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Reano, A.F.; Domenek, S.; Pernes, M.; Beaugrand, J.; Allais, F. Ferulic acid-based bis/trisphenols as renewable antioxidants for polypropylene and poly(butylene succinate). ACS Sustain. Chem. Eng. 2016, 4, 6562–6571. [Google Scholar] [CrossRef]
- Reano, A.F.; Chérubin, J.; Peru, A.M.M.; Wang, Q.; Clément, T.; Domenek, S.; Allais, F. Structure–activity relationships and structural design optimization of a series of p-hydroxycinnamic acids-based bis-and trisphenols as novel sustainable antiradical/antioxidant additives. ACS Sustain. Chem. Eng. 2015, 3, 3486–3496. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala, B.; Averous, L.; Boquillon, N. Antioxidant properties of lignin in polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Cruz Figueroa-Espinoza, M.; Laguerre, M.; Villeneuve, P.; Lecomte, J. From phenolics to phenolipids: Optimizing antioxidants in lipid dispersions. Lipid Technol. 2013, 25, 131–134. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.-C.; Villeneuve, P. Phenolic acids enzymatic lipophilization. J. Agric. Food Chem. 2005, 53, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Jakovetić Tanasković, S.; Jokić, B.; Grbavčić, S.; Drvenica, I.; Prlainović, N.; Luković, N.; Knežević-Jugović, Z. Immobilization of Candida antarctica lipase B on kaolin and its application in synthesis of lipophilic antioxidants. Appl. Clay Sci. 2017, 135, 103–111. [Google Scholar] [CrossRef]
- Laguerre, M.; Bayrasy, C.; Lecomte, J.; Chabi, B.; Decker, E.A.; Wrutniak-Cabello, C.; Cabello, G.; Villeneuve, P. How to boost antioxidants by lipophilization? Biochimie 2013, 95, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Pion, F.; Reano, A.F.; Ducrot, P.-H.; Allais, F. Chemo-enzymatic preparation of new bio-based bis-and trisphenols: New versatile building blocks for polymer chemistry. RSC Adv. 2013, 3, 8988–8997. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Mario, P.; Rosaria, C.; Hiroshi, K.; Michele, R.; Cristina, D.P. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar]
- Reano, F.A. L’acide Férulique et la (Bio-)Catalyse: Un Tandem Efficace pour la Production de Nouveaux Antioxydants Polyphénoliques; Ecole Doctorale ABIES AgroParisTech: Paris, France, 2016. [Google Scholar]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH. free radical method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Pospíšil, J.; Nešpůrek, S.; Zweifel, H. The role of quinone methides in thermostabilization of hydrocarbon polymers—I. Formation and reactivity of quinone methides. Polym. Degrad. Stab. 1996, 54, 7–14. [Google Scholar] [CrossRef]
- Pospíšil, J. Mechanistic action of phenolic antioxidants in polymers—A review. Polym. Degrad. Stab. 1988, 20, 181–202. [Google Scholar] [CrossRef]









| Compound | δd (J1/2 cm−3/2) | δp (J1/2 cm−3/2) | δh (J1/2 cm−3/2) | HiSP (J1/2 cm−3/2) |
|---|---|---|---|---|
| BDF | 21.3 | 3.5 | 13.6 | 25.5 |
| 21.5 | 3.7 | 13.9 | 25.9 | |
| IDF | 23.3 | 7.2 | 14.9 | 28.5 |
| GDF10 | 19.8 | 2.5 | 11.7 | 23.2 |
| GDF14 | 19.5 | 2.2 | 10.9 | 22.5 |
| GDF16 | 19.3 | 2.1 | 10.7 | 22.2 |
| Irganox 1010® | 18.9 | 1.3 | 10.0 | 21.4 |
| Irganox 1076® | 17.4 | 1.3 | 7.0 | 18.8 |
| Compound | Thermostability (Td5%, °C) |
|---|---|
| GDF10 | 302 |
| GDF14 | 311 |
| GDF16 | 308 |
| Irganox1076® | 236 |
| Irganox1010® | 347 |
| Compound | Free Phenols | EC50 (nmol) | Stoichiometries (n) | ׀m(EC50)׀ | AE |
|---|---|---|---|---|---|
| GDF10 | 2 | 4.81 ± 0.17 | 4.17 ±0.15 | 2.19 | 0.45 |
| GDF14 | 2 | 5.38 ± 0.12 | 3.72 ± 0.09 | 2.30 | 0.42 |
| GDF16 | 2 | 4.66 ± 0.15 | 4.30 ± 0.14 | 2.16 | 0.46 |
| Irganox 1010® | 4 | 2.52 ± 0.16 | 7.98 ± 0.47 | 0.76 | 0.30 |
| Irganox 1076® | 1 | 11.48 ± 0.17 | 1.74 ± 0.03 | 0.68 | 0.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollande, L.; Domenek, S.; Allais, F. Chemo-Enzymatic Synthesis of Renewable Sterically-Hindered Phenolic Antioxidants with Tunable Polarity from Lignocellulose and Vegetal Oil Components. Int. J. Mol. Sci. 2018, 19, 3358. https://doi.org/10.3390/ijms19113358
Hollande L, Domenek S, Allais F. Chemo-Enzymatic Synthesis of Renewable Sterically-Hindered Phenolic Antioxidants with Tunable Polarity from Lignocellulose and Vegetal Oil Components. International Journal of Molecular Sciences. 2018; 19(11):3358. https://doi.org/10.3390/ijms19113358
Chicago/Turabian StyleHollande, Louis, Sandra Domenek, and Florent Allais. 2018. "Chemo-Enzymatic Synthesis of Renewable Sterically-Hindered Phenolic Antioxidants with Tunable Polarity from Lignocellulose and Vegetal Oil Components" International Journal of Molecular Sciences 19, no. 11: 3358. https://doi.org/10.3390/ijms19113358