A Model of Evolutionary Selection: The Cardiovascular Protective Function of the Longevity Associated Variant of BPIFB4
Abstract
:1. Introduction
2. Genome-Wide Association Studies: Healthspan Depends on a Vascular System Condition
3. Longevity Associated Variant (LAV) of Bactericidal/Permeability-Increasing Fold-Containing Family B, Member 4 (BPIFB4)
4. Aging and Cardiovascular Diseases
5. The Role of Endothelial Cells in Vascular Homeostasis
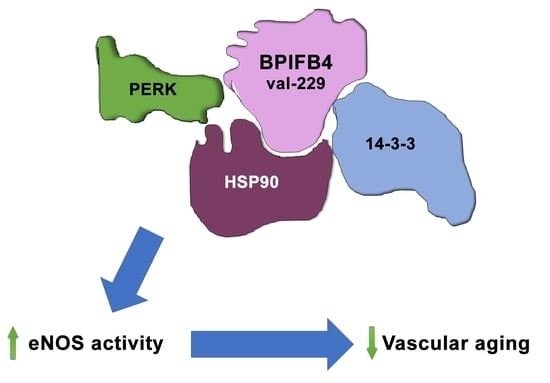
6. LAV-BPIFB4 Characterization and Its Therapeutic Potential
6.1. Structure and Localization
6.2. Molecular Functions and Cardiovascular Therapeutic Role
6.3. Endothelial Progenitor Cells Homing Enhancement
6.4. Enhancement of Calcium Mobilization
6.5. Alternative BPIFB4 Isoforms
6.6. Molecular Mechanism of BPIFB4 Isoforms
7. Therapeutic Approaches
8. Microvasculopathies
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- OECD Health at a Glance 2017. Available online: http://dx.doi.org/10.1787/health_glance-2017-en (accessed on 21 August 2018).
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Gomez-Cabrera, M.C.; Vina, J. Exercise and hormesis: Activation of cellular antioxidant signaling pathway. Ann. N. Y. Acad. Sci. 2006, 1067, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Kasapis, C.; Thompson, P.D. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Rafie, N.; Golpour Hamedani, S.; Barak, F.; Safavi, S.M.; Miraghajani, M. Dietary patterns, food groups and telomere length: A systematic review of current studies. Eur. J. Clin. Nutr. 2017, 71, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Starkweather, A.R.; Alhaeeri, A.A.; Montpetit, A.; Brumelle, J.; Filler, K.; Montpetit, M.; Mohanraj, L.; Lyon, D.E.; Jackson-Cook, C.K. An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs. Res. 2014, 63, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Andrew, T.; Gardner, J.P.; Kimura, M.; Oelsner, E.; Cherkas, L.F.; Aviv, A.; Spector, T.D. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Georgousopoulou, E.N.; Pitsavos, C.; Chrysohoou, C.; Metaxa, V.; Georgiopoulos, G.A.; Kalogeropoulou, K.; Tousoulis, D.; Stefanadis, C. Ten-year (2002–2012) cardiovascular disease incidence and all-cause mortality, in urban Greek population: The ATTICA Study. Int. J. Cardiol. 2015, 180, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kollia, N.; Panagiotakos, D.B.; Georgousopoulou, E.; Chrysohoou, C.; Tousoulis, D.; Stefanadis, C.; Papageorgiou, C.; Pitsavos, C. Exploring the association between low socioeconomic status and cardiovascular disease risk in healthy Greeks, in the years of financial crisis (2002–2012): The ATTICA study. Int. J. Cardiol. 2016, 223, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Strait, J.B.; Lakatta, E.G. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail. Clin. 2012, 8, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Sanchez, H.; Soneji, S.; Crimmins, E.M. Past, Present, and Future of Healthy Life Expectancy. Cold Spring Harb. Perspect. Med. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Population Ageing 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Kollia, N.; Tragaki, A.; Syngelakis, A.I.; Panagiotakos, D. Trends of Cardiovascular Disease Mortality in Relation to Population Aging in Greece (1956–2015). Open Cardiovasc. Med. J. 2018, 12, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Perls, T.T.; Wilmoth, J.; Levenson, R.; Drinkwater, M.; Cohen, M.; Bogan, H.; Joyce, E.; Brewster, S.; Kunkel, L.; Puca, A. Life-long sustained mortality advantage of siblings of centenarians. Proc. Natl. Acad. Sci. USA 2002, 99, 8442–8447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Lunetta, K.L.; Zhao, Q.; Mandaviya, P.R.; Rong, J.; Benjamin, E.J.; Joehanes, R.; Levy, D.; van Meurs, J.B.J.; Larson, M.G.; et al. Whole Blood Gene Expression Associated with Clinical Biological Age. J. Gerontol. A Biol. Sci. Med. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Solovieff, N.; Dewan, A.T.; Walsh, K.M.; Puca, A.; Hartley, S.W.; Melista, E.; Andersen, S.; Dworkis, D.A.; Wilk, J.B.; et al. Genetic signatures of exceptional longevity in humans. PLoS ONE 2012, 7, e29848. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Gurinovich, A.; Nygaard, M.; Sasaki, T.; Sweigart, B.; Bae, H.; Andersen, S.L.; Villa, F.; Atzmon, G.; Christensen, K.; et al. APOE Alleles and Extreme Human Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.O.; Vannice, G.K.; Rosa Neto, J.C.; Calder, P.C. Is Palmitoleic Acid a Plausible Nonpharmacological Strategy to Prevent or Control Chronic Metabolic and Inflammatory Disorders? Mol. Nutr. Food Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Koulman, A.; Griffin, J.L. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: Progress from the metabolome. Lancet Diabetes Endocrinol. 2014, 2, 65–75. [Google Scholar] [CrossRef]
- Appiah, D.; Baumgartner, R.N. The Influence of Education and Apolipoprotein epsilon4 on Mortality in Community-Dwelling Elderly Men and Women. J. Aging Res. 2018, 2018, 6037058. [Google Scholar] [CrossRef] [PubMed]
- Poirier, J.; Miron, J.; Picard, C.; Gormley, P.; Theroux, L.; Breitner, J.; Dea, D. Apolipoprotein E and lipid homeostasis in the etiology and treatment of sporadic Alzheimer’s disease. Neurobiol. Aging 2014, 35 (Suppl. S2), S3–S10. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Kato, T.; Atsumi, A.; Yamamoto, T.; Inoue, N.; Ishikawa, M.; Okada, S.; Ishigaki, N.; et al. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat. Med. 2007, 13, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Viviani Anselmi, C.; Ferreri, C.; Novelli, V.; Roncarati, R.; Bronzini, R.; Marchese, G.; Somalvico, F.; Condorelli, G.; Montenero, A.S.; Puca, A.A. Fatty acid percentage in erythrocyte membranes of atrial flutter/fibrillation patients and controls. J. Interv. Card. Electrophysiol. 2010, 27, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Kelly, M.A.; Abbott, S.K. Polyunsaturated fats, membrane lipids and animal longevity. J. Comp. Physiol. B 2014, 184, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Pol, T.; Held, C.; Westerbergh, J.; Lindback, J.; Alexander, J.H.; Alings, M.; Erol, C.; Goto, S.; Halvorsen, S.; Huber, K.; et al. Dyslipidemia and Risk of Cardiovascular Events in Patients with Atrial Fibrillation Treated with Oral Anticoagulation Therapy: Insights from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) Trial. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Rizza, S.; Cardaci, S.; Montagna, C.; Di Giacomo, G.; De Zio, D.; Bordi, M.; Maiani, E.; Campello, S.; Borreca, A.; Puca, A.A.; et al. S-nitrosylation drives cell senescence and aging in mammals by controlling mitochondrial dynamics and mitophagy. Proc. Natl. Acad. Sci. USA 2018, 115, E3388–E3397. [Google Scholar] [CrossRef] [PubMed]
- Hasler, R.; Venkatesh, G.; Tan, Q.; Flachsbart, F.; Sinha, A.; Rosenstiel, P.; Lieb, W.; Schreiber, S.; Christensen, K.; Christiansen, L.; et al. Genetic interplay between human longevity and metabolic pathways—A large-scale eQTL study. Aging Cell 2017, 16, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, R.; Ciaglia, E.; Amodio, G.; Picardi, P.; Proto, M.C.; Gazzerro, P.; Laezza, C.; Remondelli, P.; Bifulco, M.; Pisanti, S. N6-isopentenyladenosine dual targeting of AMPK and Rab7 prenylation inhibits melanoma growth through the impairment of autophagic flux. Cell Death Differ. 2017, 25, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Carrizzo, A.; Spinelli, C.C.; Ferrario, A.; Malovini, A.; Maciag, A.; Damato, A.; Auricchio, A.; Spinetti, G.; Sangalli, E.; et al. Genetic Analysis Reveals a Longevity-Associated Protein Modulating Endothelial Function and Angiogenesis. Circ. Res. 2015, 117, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Nebel, A.; Croucher, P.J.; Stiegeler, R.; Nikolaus, S.; Krawczak, M.; Schreiber, S. No association between microsomal triglyceride transfer protein (MTP) haplotype and longevity in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 7906–7909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novelli, V.; Viviani Anselmi, C.; Roncarati, R.; Guffanti, G.; Malovini, A.; Piluso, G.; Puca, A.A. Lack of replication of genetic associations with human longevity. Biogerontology 2008, 9, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bettaga, N.; Jager, R.; Dunnes, S.; Groneberg, D.; Friebe, A. Cell-specific impact of nitric oxide-dependent guanylyl cyclase on arteriogenesis and angiogenesis in mice. Angiogenesis 2015, 18, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Carrizzo, A.; Alfano, A.; Virtuoso, N.; Capunzo, M.; Calabrese, M.; De Simone, E.; Sciarretta, S.; Frati, G.; Oliveti, M.; et al. The Impact of Aging on Cardio and Cerebrovascular Diseases. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Lichtman, A.H. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, R.M.; Nahrendorf, M.; Riksen, N.P.; Netea, M.G.; de Winther, M.P.J.; Lutgens, E.; Nordestgaard, B.; Neidhart, M.; Stroes, E.S.G.; Catapano, A.L.; et al. Monocyte and haematopoietic progenitor reprogramming as common mechanism underlying chronic inflammatory and cardiovascular diseases. Eur. Heart J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Spinelli, C.C.; Martucciello, S.; Nori, S.L.; Capunzo, M.; Puca, A.A.; Ciaglia, E. Innate immunity and cellular senescence: The good and the bad in the developmental and aged brain. J. Leukoc. Biol. 2018, 103, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Puca, A.A.; Carrizzo, A.; Ferrario, A.; Villa, F.; Vecchione, C. Endothelial nitric oxide synthase, vascular integrity and human exceptional longevity. Immun. Ageing 2012, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puca, A.A.; Spinetti, G.; Vono, R.; Vecchione, C.; Madeddu, P. The genetics of exceptional longevity identifies new druggable targets for vascular protection and repair. Pharmacol. Res. 2016, 114, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amodio, G.; Moltedo, O.; Faraonio, R.; Remondelli, P. Targeting the Endoplasmic Reticulum Unfolded Protein Response to Counteract the Oxidative Stress-Induced Endothelial Dysfunction. Oxidative Med. Cell. Longev. 2018, 2018, 4946289. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Wang, L.; Qian, L. The role of eNOS in the migration and proliferation of bone-marrow derived endothelial progenitor cells and in vitro angiogenesis. Cell Biol. Int. 2015, 39, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Intracellular calcium homeostasis and signaling. Met. Ions Life Sci. 2013, 12, 119–168. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Ottolini, D.; Cali, T.; Carafoli, E. Calcium in health and disease. Met. Ions Life Sci. 2013, 13, 81–137. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, J.; Bledsoe, G.; Chao, L. Protective Role of Kallistatin in Vascular and Organ Injury. Hypertension 2016, 68, 533–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakuchi, M.; Hashiguchi, T. Endothelial Cell Aging: How miRNAs Contribute? J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Losordo, D.; Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 2012, 110, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Virtue, A.; Shen, J.; Wang, H.; Yang, X.F. An evolving new paradigm: Endothelial cells—Conditional innate immune cells. J. Hematol. Oncol. 2013, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Bingle, C.D.; Craven, C.J. PLUNC: A novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Hum. Mol. Genet. 2002, 11, 937–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, I.A.; D’Souza, R.C.; Yang, B.; Verlaan-de Vries, M.; Mann, M.; Vertegaal, A.C. Uncovering global SUMOylation signaling networks in a site-specific manner. Nat. Struct. Mol. Biol. 2014, 21, 927–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelli, C.C.; Carrizzo, A.; Ferrario, A.; Villa, F.; Damato, A.; Ambrosio, M.; Madonna, M.; Frati, G.; Fucile, S.; Sciaccaluga, M.; et al. LAV-BPIFB4 isoform modulates eNOS signalling through Ca2+/PKC-alpha-dependent mechanism. Cardiovasc. Res. 2016, 113, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Spinetti, G.; Sangalli, E.; Specchia, C.; Villa, F.; Spinelli, C.; Pipolo, R.; Carrizzo, A.; Greco, S.; Voellenkle, C.; Vecchione, C.; et al. The expression of the BPIFB4 and CXCR4 associates with sustained health in long-living individuals from Cilento-Italy. Aging 2017, 9, 370–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, F.; Malovini, A.; Carrizzo, A.; Spinelli, C.C.; Ferrario, A.; Maciag, A.; Madonna, M.; Bellazzi, R.; Milanesi, L.; Vecchione, C.; et al. Serum BPIFB4 levels classify health status in long-living individuals. Immun. Ageing 2015, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, C.; Villa, F.; Carrizzo, A.; Spinelli, C.C.; Damato, A.; Ambrosio, M.; Ferrario, A.; Madonna, M.; Uccellatore, A.; Lupini, S.; et al. A rare genetic variant of BPIFB4 predisposes to high blood pressure via impairment of nitric oxide signaling. Sci. Rep. 2017, 7, 9706. [Google Scholar] [CrossRef] [PubMed]
- Rohlenova, K.; Veys, K.; Miranda-Santos, I.; De Bock, K.; Carmeliet, P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol. 2017, 28, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Farrow, K.N.; Lakshminrusimha, S.; Reda, W.J.; Wedgwood, S.; Czech, L.; Gugino, S.F.; Davis, J.M.; Russell, J.A.; Steinhorn, R.H. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L979–987. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.N.; Wilkins, M.R.; Zhao, L. Tetrahydrobiopterin and the regulation of hypoxic pulmonary vasoconstriction. Eur. Respir. J. 2010, 36, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, J.; Hedegaard, E.R.; Simonsen, U.; Kruger, M.; Infanger, M.; Grimm, D. Current and Future Treatments for Persistent Pulmonary Hypertension in the Newborn. Basic Clin. Pharmacol. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.; Nakano, A.; Tanaka, S.; Yanagi, K.; Ohta, S.; Jojima, T.; Kasai, K.; Takekawa, H.; Hirata, K.; Hattori, Y. Fluvastatin upregulates endothelial nitric oxide synthase activity via enhancement of its phosphorylation and expression and via an increase in tetrahydrobiopterin in vascular endothelial cells. Int. J. Cardiol. 2012, 156, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Patel, M.; Yum, K.; Keyzner, A. Hematopoietic stem cell transplant-associated thrombotic microangiopathy: Review of pharmacologic treatment options. Transfusion 2015, 55, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Sadati, S.M.; Radfar, M.; Hamidi, A.K.; Abdollahi, M.; Qorbani, M.; Esfahani, E.N.; Amoli, M.M. Association Between the Polymorphism of Glu298Asp in Exon 7 of the eNOS Gene with Foot Ulcer and Oxidative Stress in Adult Patients with Type 2 Diabetes. Can. J. Diabetes 2017, 42, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, D.; Wang, F.; Hou, Z.; Fang, Z. In situ eNOS/NO up-regulation-a simple and effective therapeutic strategy for diabetic skin ulcer. Sci. Rep. 2016, 6, 30326. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E. Vascular endothelial growth factor antagonist therapy for retinopathy of prematurity. Clin. Perinatol. 2014, 41, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 2015, 122, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.D. Insights in ROP. Am. Orthopt. J. 2014, 64, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Chan-Ling, T.; Gole, G.A.; Quinn, G.E.; Adamson, S.J.; Darlow, B.A. Pathophysiology, screening and treatment of ROP: A multi-disciplinary perspective. Prog. Retin. Eye Res. 2017, 62, 77–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lien, R.; Wang, N.K.; Chao, A.N.; Chen, K.J.; Chen, T.L.; Hwang, Y.S.; Lai, C.C.; Wu, W.C. Changes in systemic vascular endothelial growth factor levels after intravitreal injection of aflibercept in infants with retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.S.; Sun, Y.; Gantner, M.L.; Shao, Z.; Evans, L.P.; Saba, N.; Fredrick, T.; Burnim, S.; Kim, J.S.; Patel, G.; et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med. 2016, 22, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthiaume, A.A.; Hartmann, D.A.; Majesky, M.W.; Bhat, N.R.; Shih, A.Y. Pericyte Structural Remodeling in Cerebrovascular Health and Homeostasis. Front. Aging Neurosci. 2018, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.O.; Garman, K.A.; Lee, Y.G.; Zuo, C.; Beck, P.J.; Tan, M.; Aguilar-Pimentel, J.A.; Ollert, M.; Schmidt-Weber, C.; Fuchs, H.; et al. The Role of Fibroblast Growth Factor-Binding Protein 1 in Skin Carcinogenesis and Inflammation. J. Investig. Dermatol. 2017, 138, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Gemenetzi, M.; Lotery, A.J. The role of epigenetics in age-related macular degeneration. Eye 2014, 28, 1407–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maugeri, A.; Barchitta, M.; Mazzone, M.G.; Giuliano, F.; Basile, G.; Agodi, A. Resveratrol Modulates SIRT1 and DNMT Functions and Restores LINE-1 Methylation Levels in ARPE-19 Cells under Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.A.; Mintz-Hittner, H.A. Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J. Am. Assoc. Pediatr. Ophthalmol. Strab. 2017, 22, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Joyal, J.S.; Hatton, C.J.; Juan, A.M.; Pei, D.T.; Hurst, C.G.; Xu, D.; Stahl, A.; Hellstrom, A.; Smith, L.E. Propranolol inhibition of beta-adrenergic receptor does not suppress pathologic neovascularization in oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Ziylan, S.; Ozturk, V.; Yabas-Kiziloglu, O.; Ciftci, F. Myopia, visual acuity and strabismus in the long term following treatment of retinopathy of prematurity. Turk. J. Pediatr. 2014, 56, 518–523. [Google Scholar] [PubMed]
- Sato, T.; Wada, K.; Arahori, H.; Kuno, N.; Imoto, K.; Iwahashi-Shima, C.; Kusaka, S. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am. J. Ophthalmol. 2011, 153, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K.S.; Galvin, O.M.; Collins, A.; Katusic, Z.S.; McDonald, D.M. BH4-Mediated Enhancement of Endothelial Nitric Oxide Synthase Activity Reduces Hyperoxia-Induced Endothelial Damage and Preserves Vascular Integrity in the Neonate. Investig. Ophthalmol. Vis. Sci. 2017, 58, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, E.E.; O’Brien, T. Isolation of circulating angiogenic cells. Methods Mol. Biol. 2012, 916, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.P.; Lee, D.; Lee, S.H.; Lee, H.E.; Lee, H.Y.; Lee, Y.M. Deguelin inhibits vasculogenic function of endothelial progenitor cells in tumor progression and metastasis via suppression of focal adhesion. Oncotarget 2015, 6, 16588–16600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, T.L.P.; Li Calzi, S.; Shaw, L.C.; Yoder, M.C.; Grant, M.B. Promoting vascular repair in the retina: Can stem/progenitor cells help? Eye Brain 2016, 8, 113–122. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa, F.; Carrizzo, A.; Ferrario, A.; Maciag, A.; Cattaneo, M.; Spinelli, C.C.; Montella, F.; Damato, A.; Ciaglia, E.; Puca, A.A. A Model of Evolutionary Selection: The Cardiovascular Protective Function of the Longevity Associated Variant of BPIFB4. Int. J. Mol. Sci. 2018, 19, 3229. https://doi.org/10.3390/ijms19103229
Villa F, Carrizzo A, Ferrario A, Maciag A, Cattaneo M, Spinelli CC, Montella F, Damato A, Ciaglia E, Puca AA. A Model of Evolutionary Selection: The Cardiovascular Protective Function of the Longevity Associated Variant of BPIFB4. International Journal of Molecular Sciences. 2018; 19(10):3229. https://doi.org/10.3390/ijms19103229
Chicago/Turabian StyleVilla, Francesco, Albino Carrizzo, Anna Ferrario, Anna Maciag, Monica Cattaneo, Chiara Carmela Spinelli, Francesco Montella, Antonio Damato, Elena Ciaglia, and Annibale Alessandro Puca. 2018. "A Model of Evolutionary Selection: The Cardiovascular Protective Function of the Longevity Associated Variant of BPIFB4" International Journal of Molecular Sciences 19, no. 10: 3229. https://doi.org/10.3390/ijms19103229