The Antioxidant and Anti-Inflammatory Activities of 8-Hydroxydaidzein (8-HD) in Activated Macrophage-Like RAW264.7 Cells
Abstract
:1. Introduction
2. Results
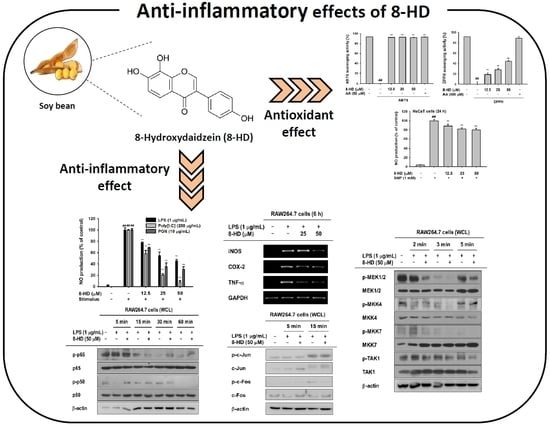
2.1. Antioxidant Effects of 8-HD
2.2. Effect of 8-HD on Nitric Oxide Production
2.3. Anti-Inflammatory Effects of 8-HD at the Transcriptional Level
2.4. Anti-Inflammatory Effects of 8-HD on NF-κB and AP-1 Signaling
3. Discussion
4. Materials and Methods
4.1. Biochemical Reagents
4.2. Cell Culture
4.3. DPPH Assays
4.4. ABTS Assays
4.5. NO Production and Griess Assays
4.6. Cell Viability Assays
4.7. Preparation of mRNA and Semi-Quantitative PCR
4.8. Plasmid Transfections
4.9. Preparation of Whole Cell Lysates and Immunoblotting
4.10. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 8-HD | 8-hydroxydaidzein |
| SNP | sodium nitropursside |
| ROS | radical oxygen species |
| iNOS | inducible nitric oxide synthase |
| COX | cycloocygenase |
| TNF | tumor necrosis factor |
| PAMPs | pattern-associated molecular patterns |
| PRRs | pattern-recognition receptors |
| NLRs | NOD-like receptors |
| RLRs | RIG-I-like receptors |
| TLRs | Toll-like receptors |
| MyD88 | myeloid differentiation primary response 88 |
| TRIF | TIR domain-containing adaptor-including interferon-β |
| IKK | IκB kinase |
| IL | interleukin |
| MAPKs | mitogen-activated protein kinases |
| ERK | extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| MEK | MAPK/ERK kinase |
| MKK | mitogen-activated protein kinase kinase |
| TAK1 | transforming growth factor beta-activated kinase 1 |
| GSK | glycogen synthase kinase |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| LPS | lipopolysaccharide |
| poly[I:C] | polyinosinic-polycytidylic acid |
| PGN | peptidoglycan |
| NO | nitric oxide |
| L-NAME | NG-nitro-L-arginine methyl ester |
| PEI | polyethylenimine |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| FBS | fetal bovine serum |
| EDTA | ethylenediaminetetraacetic acid |
| EGTA | ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| TLA | three-letter acronym |
| LD | linear dichroism |
References
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Shaw, S.G.; Shi-Wen, X.; Abraham, D.; Baker, D.M.; Tsui, J. Toll-like receptors in ischaemia and its potential role in the pathophysiology of muscle damage in critical limb ischaemia. Cardiol. Res. Pract. 2012, 2012, 121237. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Ann. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.; O’Neill, L.A. The interleukin-1 receptor/Toll-like receptor superfamily: Signal generators for pro-inflammatory interleukins and microbial products. J. Leukoc. Biol. 2000, 67, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Gerer, K.F.; Erdmann, M.; Hadrup, S.R.; Lyngaa, R.; Martin, L.M.; Voll, R.E.; Schuler-Thurner, B.; Schuler, G.; Schaft, N.; Hoyer, S.; et al. Preclinical evaluation of NF-kappaB-triggered dendritic cells expressing the viral oncogenic driver of Merkel cell carcinoma for therapeutic vaccination. Ther. Adv. Med. Oncol. 2017, 9, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Ann. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816. [Google Scholar] [CrossRef] [PubMed]
- Foletta, V.C.; Segal, D.H.; Cohen, D.R. Transcriptional regulation in the immune system: All roads lead to AP-1. J. Leukoc. Biol. 1998, 63, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Kim, S.C.; Son, Y.-J.; Baek, K.-S.; Kim, E.; Yang, W.S.; Jeong, D.; Park, J.G.; Kim, H.G.; Chung, W.-J. AP-1-targeting anti-inflammatory activity of the methanolic extract of Persicaria chinensis. Evid. Based Complement. Altern. Med. 2015, 2015, 608126. [Google Scholar]
- Kaufman, P.B.; Duke, J.A.; Brielmann, H.; Boik, J.; Hoyt, J.E. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: Implications for human nutrition and health. J. Altern. Complement. Med. 1997, 3, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-H.; Kim, B.-N.; Kim, K.-R.; Lee, K.W.; Lee, C.-H.; Oh, D.-K. Production of 8-hydroxydaidzein from soybean extract by Aspergillus oryzae KACC 40247. Biosci. Biotechnol. Biochem. 2013, 77, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-M.; Ding, H.-Y.; Tsai, Y.-T.; Chang, T.-S. Production of two novel methoxy-isoflavones from biotransformation of 8-hydroxydaidzein by recombinant Escherichia coli expressing O-methyltransferase SpOMT2884 from Streptomyces peucetius. Int. J. Mol. Sci. 2015, 16, 27816–27823. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Funako, T.; Hayashi, H. 8-Hydroxydaidzein, an aldose reductase inhibitor from okara fermented with Aspergillus sp. HK-388. Biosci. Biotechnol. Biochem. 2004, 68, 1588–1590. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; De Pascual-Teresa, S.; Ewins, B.; Matsugo, S.; Uchida, Y.; Minihane, A.; Turner, R.; Vafei Adou, K.; Weinberg, P. Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiotica 2003, 33, 913–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esaki, H.; Shirasaki, T.; Yamashita, K.; Nakamura, Y.; Kawakishi, S.; Osawa, T. Absorption and excretion of the 8-hydroxydaidzein in rats after oral administration and its antioxidant effect. J. Nutr. Sci. Vitaminol. 2005, 51, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Park, H.Y.; Kim, D.H.; Kim, D.H.; Kim, H.K. ortho-Dihydroxyisoflavone derivatives from aged Doenjang (Korean fermented soypaste) and its radical scavenging activity. Bioorg. Med. Chem. Lett. 2008, 18, 5006–5009. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Chang, C.-W.; Lin, C.-W.; Hsu, Y.-C. Production of 8-hydroxydaidzein polyphenol using biotransformation by Aspergillus oryzae. Food Sci. Technol. Res. 2015, 21, 557–562. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, D.H.; Lee, J.K.; Lee, J.Y.; Kim, D.H.; Kim, H.K.; Lee, H.-J.; Kim, H.C. Natural ortho-dihydroxyisoflavone derivatives from aged Korean fermented soybean paste as potent tyrosinase and melanin formation inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, C.J.; Loke, T.-K. Clinical and molecular aspects of glucocorticoid resistant asthma. Ther. Clin. Risk Manag. 2007, 3, 771–787. [Google Scholar] [PubMed]
- Tas, S.W.; Maracle, C.X.; Balogh, E.; Szekanecz, Z. Targeting of proangiogenic signalling pathways in chronic inflammation. Nat. Rev. Rheumatol. 2016, 12, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mohan, C. Toll-like receptor signaling pathways—Therapeutic opportunities. Mediat. Inflamm. 2010, 2010, 781235. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Cao, X. Regulation of Toll-like receptor signaling pathways in innate immune responses. Ann N. Y. Acad. Sci. 2013, 1283, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, P.; Richmond, A. A novel NF-κB-inducing kinase-MAPK signaling pathway up-regulates NF-κB activity in melanoma cells. J. Biol. Chem. 2002, 277, 7920–7928. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Ferrari, D.; Riehemann, K.; Wesselborg, S. Regulation of NF-κB activation by MAP kinase cascades. Immunobiology 1997, 198, 35–49. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The regulation of NF-κB subunits by phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, J.G.; Hong, Y.D.; Kim, E.; Baik, K.-S.; Yoon, D.H.; Kim, S.; Lee, M.-N.; Rho, H.S.; Shin, S.S. Src/Syk/IRAK1-targeted anti-inflammatory action of Torreya nucifera butanol fraction in lipopolysaccharide-activated RAW264.7 cells. J. Ethnopharmacol. 2016, 188, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.-B.; Oh, H.-K.; Yu, S.A.; Moon, S.H.; Choe, E.K.; Oh, T.Y.; Park, K.J. The effects of eupatilin (stillen®) on motility of human lower gastrointestinal tracts. Korean J. Physiol. Pharmacol. 2014, 18, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Corrêa, M.A.; Chorilli, M. Sustainability, natural and organic cosmetics: Consumer, products, efficacy, toxicological and regulatory considerations. Braz. J. Pharm. Sci. 2015, 51, 17–26. [Google Scholar] [CrossRef]
- Culasso, B. Natural Cosmetics and Consumer Touchpoints, the Valuable Factors Shaping the Shopping Experience. Master’s Thesis, Copenhagen Business School, Frederiksberg, Danmark, 2014. [Google Scholar]
- Ghimeray, A.K.; Lee, H.Y.; Kim, Y.H.; Ryu, E.K.; Chang, M.S. Evaluation of antioxidant and anti-inflammatory effect of Rhododendron brachycarpum extract used in skin care product by In Vitro and In Vivo test. Technol. Invest. 2015, 6, 105–111. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J.; Pytkowska, K. Moisturizing and antiinflammatory properties of cosmetic formulations containing Centella asiatica extract. Indian J. Pharm. Sci. 2016, 78, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Brönstrup, M. Industrial natural product chemistry for drug discovery and development. Nat. Prod. Rep. 2014, 31, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Amirkia, V.; Heinrich, M. Natural products and drug discovery: A survey of stakeholders in industry and academia. Front. Pharmacol. 2015, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Hong, Y.D.; Baek, K.-S.; Yoo, S.; Hong, Y.H.; Kim, J.H.; Lee, J.O.; Kim, D.; Park, J.; Cho, J.Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J. Ginseng Res. 2017, 41, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Hong, J.T.; Han, S.B.; Park, Y.H.; Son, D.J. Effect of Ixeris dentata Nakai extract on nitric oxide production and prostaglandin E2 generation in LPS-stimulated RAW264. 7 Cells. Immune Netw. 2015, 15, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.-S.; Yi, Y.-S.; Son, Y.-J.; Yoo, S.; Sung, N.Y.; Kim, Y.; Hong, S.; Aravinthan, A.; Kim, J.-H.; Cho, J.Y. In Vitro and In Vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J. Ginseng Res. 2016, 40, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Burnette, W.N. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Kang, Y.-G.; Kim, J.H.; Kim, Y.-J.; Lee, T.R.; Lee, J.; Kim, D.; Cho, J.Y. The Antioxidant and Anti-Inflammatory Activities of 8-Hydroxydaidzein (8-HD) in Activated Macrophage-Like RAW264.7 Cells. Int. J. Mol. Sci. 2018, 19, 1828. https://doi.org/10.3390/ijms19071828
Kim E, Kang Y-G, Kim JH, Kim Y-J, Lee TR, Lee J, Kim D, Cho JY. The Antioxidant and Anti-Inflammatory Activities of 8-Hydroxydaidzein (8-HD) in Activated Macrophage-Like RAW264.7 Cells. International Journal of Molecular Sciences. 2018; 19(7):1828. https://doi.org/10.3390/ijms19071828
Chicago/Turabian StyleKim, Eunji, Young-Gyu Kang, Ji Hye Kim, Yong-Jin Kim, Tae Ryong Lee, Jongsung Lee, Donghyun Kim, and Jae Youl Cho. 2018. "The Antioxidant and Anti-Inflammatory Activities of 8-Hydroxydaidzein (8-HD) in Activated Macrophage-Like RAW264.7 Cells" International Journal of Molecular Sciences 19, no. 7: 1828. https://doi.org/10.3390/ijms19071828