Therapeutics for Inflammatory-Related Diseases Based on Plasmon-Activated Water: A Review
Abstract
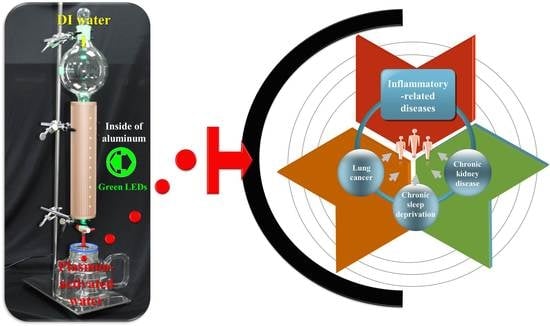
:1. Introduction
2. Results and Discussion
2.1. Creation and Characteristics of Plasmon-Activated Water (PAW)
2.2. Physchemical and Cellular Antioxidation Properties of PAW
2.3. Innovative Therapeutic Strategy Utilizing PAW
3. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Jeon, K.S.; Hwang, J.H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, J.; Han, G.; Zhao, T.; Zhang, R.; Liu, B.; Liu, Z.; Zhang, C.; Yang, L.; Zhang, Z. Click-functionalized SERS nanoprobes with improved labeling efficiency and capability for cancer cell imaging. ACS Appl. Mater. Interfaces 2017, 9, 38222–38229. [Google Scholar] [CrossRef] [PubMed]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Rebekah, A.; Drezek, R.A.; West, J.L. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cheng, Y.; Feng, Y.; Jian, H.; Wang, L.; Ma, X.; Li, X.; Zhang, H. Resonance energy transfer-promoted photothermal and photodynamic performance of gold–copper sulfide yolk–shell nanoparticles for chemophototherapy of cancer. Nano Lett. 2018, 18, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, J.; Ryu, S.; Kwon, Y.; Kim, K.H.; Jeong, D.H.; Paik, S.R.; Kim, B.S. Gold nanoparticle/graphene oxide hybrid sheets attached on mesenchymal stem cells for effective photothermal cancer therapy. Chem. Mater. 2017, 29, 3461–3476. [Google Scholar] [CrossRef]
- Kim, H.S.; Son, Y.J.; Mao, W.; Leong, K.W.; Yoo, H.S. Atom transfer radical polymerization of multishelled cationic corona for the systemic delivery of siRNA. Nano Lett. 2018, 18, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zheng, W.; Gao, L.; Cai, P.; Liu, R.; Wang, Y.; Yuan, Q.; Zhao, Y.; Gao, X. Au nanoclusters and photosensitizer dual loaded spatiotemporal controllable liposomal nanocomposites enhance tumor photodynamic therapy effect by inhibiting thioredoxin reductase. Adv. Healthc. Mater. 2017, 6, 1601453. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Xiong, X.; Chakraborty, P.K.; Shameer, K.; Arvizo, R.R.; Kudgus, R.A.; Dwivedi, S.K.D.; Hossen, M.N.; Gillies, E.M.; Robertson, J.D.; et al. Gold nanoparticle reprograms pancreatic tumor microenvironment and inhibits tumor growth. ACS Nano 2016, 10, 10636–10651. [Google Scholar] [CrossRef] [PubMed]
- Melamed, J.R.; Riley, R.S.; Valcourt, D.M.; Day, E.S. Using Gold nanoparticles to disrupt the tumor microenvironment: An emerging therapeutic strategy. ACS Nano 2016, 10, 10631–10635. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Wu, Y.; Ghosh, D.; Do, B.H.; Chen, K.; Dawson, M.R.; Fang, N.; Sulchek, T.A.; El-Sayed, M.A. Nuclear membrane-targeted gold nanoparticles inhibit cancer cell migration and invasion. ACS Nano 2017, 11, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Gatta, D.M.P.; Pesce, M.; Ferrone, A.; Martino, G.D.; Nicola, M.D.; Lutiis, M.A.D.; Vitacolonna, E.; Patruno, A.; Grilli, A.; et al. Modulation of the oxidative plasmatic state in gastroesophageal reflux disease with the addition of rich water molecular hydrogen: A new biological vision. J. Cell. Mol. Med. 2018, 22, 2750–2759. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sato, T.; Sugimoto, M.; Baskoro, H.; Karasutani, K.; Mitsui, A.; Nurwidya, F.; Arano, N.; Kodama, Y.; Hirano, S.; et al. Hydrogen-rich pure water prevents cigarette smoke-induced pulmonary emphysema in SMP30 knockout mice. Biochem. Biophys. Res. Commun. 2017, 492, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Iketani, M.; Ohshiro, J.; Urushibara, T.; Takahashi, M.; Arai, T.; Kawaguchi, H.; Ohsawa, I. Preadministration of Hydrogen-rich water protects against lipopolysaccharide-induced sepsis and attenuates liver injury. Shock 2017, 48, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Pan, W.; Zhang, J.; Xu, X.; Zhang, X.; He, X.; Fan, M. Hydrogen rich water attenuates renal injury and fibrosis byregulation transforming growth factor-β induced Sirt1. Biol. Pharm. Bull. 2017, 40, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Huang, W.N.; Li, H.H.; Huang, C.N.; Hsieh, S.; Lai, C.; Lu, F.J. Hydrogen-rich water attenuates amyloid b-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem.-Biol. Interact. 2015, 240, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.T.; Huang, T.S.; Chiu, C.C.; Pan, J.L.; Liang, S.S.; Chen, B.H.; Chen, S.H.; Liu, P.L.; Wang, H.C.; Wen, Z.H.; et al. Biological properties of acidic cosmetic water from seawater. Int. J. Mol. Sci. 2012, 13, 5952–5971. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.; Benvenuti, F.; Nappi, G.; Fortunati, N.A.; Marino, L.; Aureli, T.; De Luca, S.; Pagliarani, S.; Canestrari, F. Antioxidative effects of sulfurous mineral water: Protection against lipid and protein oxidation. Eur. J. Clin. Nutr. 2009, 63, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Sasso, M.D.; Culici, M.; Falchi, M.; Spallino, A.; Nappi, G. Free radical–scavenging activity of sulfurous water investigated by electron paramagnetic resonance (EPR) spectroscopy. Exp. Lung Res. 2012, 38, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Shultz, M.J.; Vu, T.H.; Meyer, B.; Bisson, P. Water: A responsive small molecule. Acc. Chem. Res. 2012, 45, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Hwang, B.J.; Mai, F.D.; Liu, Y.C.; Lin, C.M.; Kuo, H.S.; Chou, D.S.; Lee, M.J.; Yang, K.H.; Yu, C.C.; et al. Active and stable liquid water innovatively prepared using resonantly illuminated gold nanoparticles. ACS Nano 2014, 8, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, X.; Zhang, J.Z.H.; Qi, L.W. Hydrogen-bond structure dynamics in bulk water: Insights from ab initio simulations with coupled cluster theory. ACS Nano 2018, 9, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Cappa, C.D.; Wilson, K.R.; Cohen, R.C.; Geissler, P.L.; Saykally, R.J. Unified description of temperature-dependent hydrogen-bond rearrangements in liquid water. Proc. Natl. Acad. Sci. USA 2005, 102, 14171–14174. [Google Scholar] [CrossRef] [PubMed]
- Modig, K.; Pfrommer, B.G.; Halle, B. Temperature-dependent hydrogen-bond geometry in liquid water. Phys. Rev. Lett. 2003, 90, 075502. [Google Scholar] [CrossRef] [PubMed]
- Paschek, D.; Ludwig, R. Specific ion effects on water structure and dynamics beyond the first hydration shell. Angew. Chem. Int. Ed. 2011, 50, 352–353. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Saykally, R.J.; Geissler, P.L. The effects of dissolved halide anions on hydrogen bonding in liquid water. J. Am. Chem. Soc. 2007, 129, 13847–13856. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Fayer, M.D. Hydrogen bond dynamics in aqueous NaBr solutions. Proc. Natl. Acad. Sci. USA 2007, 104, 16731–16738. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, K.A.T.; Haymet, A.D.J.; Dill, K.A. The strength of hydrogen bonds in liquid water and around nonpolar solutes. J. Am. Chem. Soc. 2000, 122, 8037–8041. [Google Scholar] [CrossRef]
- Cai, R.; Yang, H.; He, J.; Zhu, W. The effects of magnetic fields on water molecular hydrogen bonds. J. Mol. Struct. 2009, 938, 15–19. [Google Scholar] [CrossRef]
- Fayer, M.D. Dynamics of water interacting with interfaces, molecules, and ions. Acc. Chem. Res. 2012, 45, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Fenn, E.E.; Wong, D.B.; Fayer, M.D. Water dynamics at neutral and ionic interfaces. Proc. Natl. Acad. Sci. USA 2009, 106, 15243–15248. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.G.; Rankin, B.M.; Gierszal, K.P.; Ben-Amotz, D. On the cooperative formation of non-hydrogen-bonded water at molecular hydrophobic interfaces. Nat. Chem. 2013, 5, 796–802. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.A.; Shen, Y.R. Ultrafast vibrational dynamics at water interfaces. Science 2006, 313, 1945–1948. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Marcus, R.A. On the theory of organic catalysis “on water”. J. Am. Chem. Soc. 2007, 129, 5492–5502. [Google Scholar] [CrossRef] [PubMed]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 6353, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Chaban, V.V.; Prezhdo, V.V.; Prezhdo, O. Confinement by carbon nanotubes drastically alters the boiling and critical behavior of water droplets. ACS Nano 2012, 6, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Chaban, V.V.; Prezhdo, O.V. Water boiling inside carbon nanotubes: Toward efficient drug release. ACS Nano 2011, 5, 5647–5655. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kumar, H.; Dasgupta, C.; Maiti, P.K. Confined water: Structure, dynamics, and thermodynamics. Acc. Chem. Res. 2017, 50, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Pyrgiotakis, G.; Vasanthakumar, A.; Gao, Y.; Eleftheriadou, M.; Toledo, E.; DeAraujo, A.; McDevitt, J.; Han, T.; Mainelis, G.; Mitchell, R.; et al. Inactivation of foodborne microorganisms using engineered water nanostructures (EWNS). Environ. Sci. Technol. 2015, 49, 3737–3745. [Google Scholar] [CrossRef] [PubMed]
- Pyrgiotakis, G.; McDevitt, J.; Bordini, A.; Diaz, E.; Molina, R.; Watson, C.; Deloid, G.; Lenard, S.; Fix, N.; Mizuyama, Y.; et al. A chemical free, nanotechnology-based method for airborne bacterial inactivation using engineered water nanostructures. Environ. Sci. Nano 2014, 1, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Pyrgiotakis, G.; Vedantam, P.; Cirenza, C.; McDevitt, J.; Eleftheriadou, M.; Leonard, S.S.; Demokritou, P. Optimization of a nanotechnology based antimicrobial platform for food safety applications using engineered water nanostructures (EWNS). Sci. Rep. 2016, 6, 21073. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.F.; Bo, D. The changes of macroscopic features and microscopic structures of water under influence of magnetic field. Physica B 2008, 403, 3571–3577. [Google Scholar] [CrossRef]
- Pang, X.F.; Deng, B.; Tang, B. Influences of magnetic field on macroscopic properties of water. Mod. Phys. Lett. B 2012, 26, 1250069. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, E.; Kim, H.Y.; Youn, D.O.; Jung, J.; Kim, H.; Chang, Y.; Lee, W.; Shin, J.; Baek, S.; et al. Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson’s disease therapy. Nat. Nanotechnol. 2017, 12, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, S.L.; Ellis, P.R.; Jackson, E.C.; Butterworth, P.J. The effect of guar galactomannan and water availability during hydrothermal processing on the hydrolysis of starch catalysed by pancreatic á-amylase. Biochim. Biophys. Acta 2002, 1571, 55–63. [Google Scholar] [CrossRef]
- Oomori, T.; Khajavi, S.H.; Kimura, Y.; Adachi, S.; Matsuno, R. Hydrolysis of disaccharides containing glucose residue in subcritical water. Biochem. Eng. J. 2004, 18, 143–147. [Google Scholar] [CrossRef]
- Valenti, W.C.; Díaz, F.C.; Stumpf, L.; López Greco, L.S.; Viau, V.E. Effect of food shortage on growth, energetic reserves mobilization, and water quality in juveniles of the redclaw crayfish, cherax quadricarinatus, reared in groups. J. Crustac. Biol. 2014, 35, 639–646. [Google Scholar]
- Akola, J.; Jones, R.O. ATP Hydrolysis in water—A density functional study. J. Phys. Chem. B 2003, 107, 11774–11783. [Google Scholar] [CrossRef] [Green Version]
- Rivard, U.; Thomas, V.; Bruhacs, A.; Siwick, B.; Iftimie, R. Donor–bridge–acceptor proton transfer in aqueous solution. J. Phys. Chem. Lett. 2014, 5, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, M.; Gargiulo, S.; Iborra, S.; Arends, I.W.C.E.; Hollmann, F.; Corma, A. Photobiocatalytic chemistry of oxidoreductases using water as the electron donor. Nat. Commun. 2014, 5, 3145. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Spijker, P.; Voitchovsky, K. Water-induced correlation between single ions imaged at the solid–liquid interface. Nat. Commun. 2014, 5, 4400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.M.; Kim, K.S. Dynamics and structural changes of small water clusters on ionization. J. Comput. Chem. 2013, 34, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Vohringer-Martinez, E.; Hansmann, B.; Hernandez, H.; Francisco, J.S.; Troe, J.; Abel, B. Water catalysis of a radical-molecule gas-phase reaction. Science 2007, 315, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Buszek, R.J.; Torrent-Sucarrat, M.; Anglada, J.M.; Francisco, J.S. The effects of a single water on the OH + H2O2 reaction. J. Phys. Chem. A 2012, 116, 5821–5829. [Google Scholar] [CrossRef] [PubMed]
- Boucher, M.B.; Marcinkowski, M.D.; Liriano, M.L.; Murphy, C.J.; Lewis, E.A.; Jewell, A.D.; Mattera, M.F.G.; Kyriakou, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. A molecular-scale perspective of water-catalyzed methanol dehydrogenation to formaldehyde. ACS Nano 2013, 7, 6181–6187. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Miao, J.; Gao, Z.; Zhang, L.; Gao, Y.; Fan, C.; Li, D. Reactivating catalytic surface: Insights into the role of hot holes in plasmonic catalysis. Small 2018, 14, 1703510. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, Y.; Shi, L.; Zhu, Y.; Mideksa, M.F.; Hou, K.; Zhao, W.; Wang, D.; Zhao, M.; Zhang, X.; et al. Boosting hot electrons in hetero-superstructures for plasmon-enhanced catalysis. J. Am. Chem. Soc. 2017, 139, 17964–17972. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, H.; Nguyen, N.T.; Zhang, Y.; Schmuki, P.; Bi, Y. Plasmon-induced hole-depletion layer on hematite nanoflake photoanodes for highly efficient solar water splitting. App. Energy 2017, 35, 171–178. [Google Scholar] [CrossRef]
- Mukherjee, S.; Libisch, F.; Large, N.; Neumann, O.; Brown, L.V.; Cheng, J.; Lassiter, J.B.; Carter, E.A.; Nordlander, P.; Halas, N.J. Hot electrons do the impossible: Plasmon-induced dissociation of H2 on Au. Nano Lett. 2013, 13, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Zhang, M.; Zhao, L.B.; Feng, J.M.; Wu, D.Y.; Ren, B.; Tian, Z.Q. Activation of oxygen on gold and silver nanoparticles assisted by surface plasmon resonances. Angew. Chem. Int. Ed. 2014, 53, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Li, Y.; Li, M.Y.; Zhang, H.; Zhang, J. Boosting electrocatalytic hydrogen evolution by Plasmon-driven hot-electron excitation. Nanoscale 2018, 10, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Mai, F.D.; Yang, K.H.; Chen, L.Y.; Yang, C.P.; Liu, Y.C. Quantitative evaluation on activated property-tunable bulk liquid water with reduced hydrogen bonds using deconvoluted Raman spectroscopy. Anal. Chem. 2015, 87, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Mai, F.D.; Hwang, B.J.; Lee, M.J.; Chen, C.H.; Wang, S.H.; Tsai, H.Y.; Yang, C.P.; Liu, Y.C. Creation of electron-doping liquid water with reduced hydrogen bonds. Sci. Rep. 2016, 6, 22166. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Lin, H.C.; Chen, H.H.; Mai, F.D.; Liu, Y.C.; Lin, C.M.; Chang, C.C.; Tsai, H.Y.; Yang, C.P. Innovative strategy with potential to increase hemodialysis efficiency and safety. Sci. Rep. 2014, 4, 4425. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Chen, H.C.; Mai, F.D.; Yu, C.C.; Chang, C.C.; Liu, Y.C. One-step fabrication of SERS-active substrates based on plasmon-induced activated water, with improved activity and excellent reproducibility. J. Electroanal. Chem. 2015, 750, 27–35. [Google Scholar] [CrossRef]
- Hwang, B.J.; Chen, H.C.; Mai, F.D.; Tsai, H.Y.; Yang, C.P.; Rick, J.; Liu, Y.C. Innovative strategy on hydrogen evolution reaction utilizing activated liquid water. Sci. Rep. 2015, 5, 16263. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Chen, H.C.; Wang, C.C.; Tsai, P.W.; Ho, C.W.; Liu, Y.C. Effective Energy transfer via plasmon-activated high-energy water promotes its fundamental activities of solubility, ionic conductivity, and extraction at room temperature. Sci. Rep. 2015, 5, 18152. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Mai, F.D.; Yang, K.H.; Tsai, H.Y.; Yang, C.P.; Chen, C.C.; Chen, C.H.; Liu, Y.C. Environmentally friendly etching agent: Vapor from hot electron-activated liquid water. Green Chem. 2016, 18, 3098–3105. [Google Scholar] [CrossRef]
- Chen, H.C.; Mai, F.D.; Yang, K.H.; Chen, L.Y.; Yang, C.P.; Liu, Y.C. Triggering comprehensive enhancement in oxygen evolution reaction by using newly created solvent. Sci. Rep. 2016, 6, 28456. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Cheng, C.Y.; Lin, H.C.; Chen, H.H.; Chen, C.H.; Yang, C.P.; Yang, K.H.; Lin, C.M.; Lin, T.Y.; Shih, C.M.; et al. Multifunctions of excited gold nanoparticles decorated artificial kidney with efficient hemodialysis and therapeutic potential. ACS Appl. Mater. Interfaces 2016, 8, 19691–19700. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Chang, C.C.; Yang, K.H.; Mai, F.D.; Tseng, C.L.; Chen, L.Y.; Hwang, B.J.; Liu, Y.C. Polypyrrole electrode with a greater electroactive surface electrochemically polymerized in plasmon-activated water. J. Taiwan Inst. Chem. E 2018, 82, 252–260. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, Y.R.; Yang, K.H.; Yang, C.P.; Tung, K.L.; Lee, M.J.; Shih, J.H.; Liu, Y.C. In situ and real-time reduction of water molecules’ interaction for efficient water evaporation. Desalination 2018, 436, 91–97. [Google Scholar] [CrossRef]
- Chen, H.C.; Cheng, C.Y.; Chen, L.Y.; Chang, C.C.; Yang, C.P.; Mai, F.D.; Liao, W.C.; Chang, H.M.; Liu, Y.C. Plasmon-activated water effectively relieves hepatic oxidative damage resulted from chronic sleep deprivation. RSC Adv. 2018, 8, 9618–9626. [Google Scholar] [CrossRef]
- Wang, C.K.; Chen, H.C.; Fang, S.U.; Ho, C.W.; Tai, C.J.; Yang, C.P.; Liu, Y.C. Innovatively therapeutic strategy on lung cancer by daily drinking antioxidative plasmon-induced activated water. Sci. Rep. 2018, 8, 6316. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Fang, S.U.; Yang, K.H.; Tsai, H.Y.; Liu, Y.C. Effectively reducing reagent concentrations for electrochemical reactions in aqueous solutions using plasmon-activated water. J. Electroanal. Chem. 2018, 818, 44–50. [Google Scholar] [CrossRef]
- Yang, C.P.; Fang, S.U.; Yang, K.H.; Chen, H.C.; Tsai, H.Y.; Mai, F.D.; Liu, Y.C. Surface-enhanced Raman scattering-active substrate prepared with new plasmon-activated water. ACS Omega 2018, 3, 4741–4751. [Google Scholar] [CrossRef]
- David, J.G.; Gierszal, K.P.; Wang, P.; Ben-Amotz, D. Water structural transformation at molecular hydrophobic interfaces. Nature 2012, 491, 582–585. [Google Scholar]
- Li, R.; Jiang, Z.; Guan, Y.; Yang, H.; Liu, B. Effects of metal ion on the water structure studied by the Raman O[BOND]H stretching spectrum. J. Raman Spectrosc. 2009, 40, 1200–1204. [Google Scholar] [CrossRef]
- Carey, D.M.; Korenowski, G.M. Measurement of the Raman spectrum of liquid water. J. Chem. Phys. 1998, 108, 2669–2775. [Google Scholar] [CrossRef]
- Tomlinson-Phillips, J.; Davis, J.; Ben-Amotz, D. Structure and dynamics of water dangling OH bonds in hydrophobic hydration shells. Comparison of simulation and experiment. J. Phys. Chem. A 2011, 115, 6177–6183. [Google Scholar] [CrossRef] [PubMed]
- Leberman, R.; Soper, A.K. Effect of high salt concentrations on water structure. Nature 1995, 378, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Chumaevskii, N.A.; Rodnikova, M.N.; Sirotkin, D.A. Cationic effect in aqueous solutions of 1:1 electrolytes by Raman spectral data. J. Mol. Liq. 2001, 91, 81–91. [Google Scholar] [CrossRef]
- Islas, R.; Heine, T.; Merino, G. The induced magnetic field. Acc. Chem. Res. 2012, 45, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Tanigaki, T.; Takahashi, Y.; Shimakura, T.; Akashi, T.; Tsuneta, R.; Sugawara, A.; Shindo, D. Three-dimensional observation of magnetic vortex cores in stacked ferromagnetic discs. Nano Lett. 2015, 15, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, S.; Long, D.J., 2nd; Jaiswal, A.K. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr. Top. Cell. Regul. 2000, 36, 201–216. [Google Scholar] [PubMed]
- Imlay, J.A. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Shimizu, M.; Katafuchi, A.; Gruz, P.; Fujii, S.; Usui, Y.; Fuchs, R.P.; Nohmi, T. Escherichia coli DNA polymerase III is responsible for the high level of spontaneous mutations in mutT strains. Mol. Microbiol. 2012, 86, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Kwon, B.; Han, E.; Park, M.; Yang, W.; Cho, W.; Yoo, W.; Khang, G.; Lee, D. Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat. Commun. 2015, 6, 6907. [Google Scholar] [CrossRef] [PubMed]
- Kanno, N.; Tonokura, K.; Tezaki, A.; Koshi, M. Water dependence of the HO2 self reaction: Kinetics of the HO2-H2O complex. J. Phys. Chem. A 2005, 109, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Cook, R.D.; Davidson, D.F.; Hanson, R.K. A shock tube study of OH + H2O2 → H2O + HO2 and H2O2 + M → 2OH + M using laser absorption of H2O and OH. J. Phys. Chem. A 2010, 114, 5718–5727. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Chau, L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002, 8, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.L.; Yang, J.Y.; Chen, G.L.; Zhang, L.J.; Wang, L.H.; Li, J.; Wang, J.M.; Wu, C.F. RV09, a novel resveratrol analogue, inhibits NO and TNF-α production by LPS-activated microglia. Int. Immunopharmacol. 2008, 8, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, J.; Park, S.; Khang, G.; Kang, P.M.; Lee, D. Inflammation-responsive antioxidant nanoparticles based on a polymeric prodrug of vanillin. Biomacromolecules 2013, 14, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, S.M.; Lee, H.; Nedrygailov, I.I. Hot-electron-mediated surface chemistry: Toward electronic control of catalytic activity. Acc. Chem. Res. 2015, 48, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhao, P.; Rong, Z.; Li, B.; Xue, H.; You, J.; He, C.; Li, W.; He, X.; Lee, R.J.; et al. Anti-tumor efficiency of lipid -coated cisplatin nano-particles co-loaded with microRNA-375. Theranostics 2016, 6, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.Y.; Rogers, P.M.; Kelland, L.R. Transport of cisplatin and bis-acetato-ammine-dichlorocyclohexylamine platinum(IV) (JM216) in human ovarian carcinoma cell lines: Identification of a plasma membrane protein associated with cisplatin resistance. Clin. Cancer Res. 1995, 1, 981–989. [Google Scholar] [PubMed]
- Lau, J.K.C.; Ensing, B. Hydrolysis of cisplatin—A first-principles metadynamics study. Phys. Chem. Chem. Phys. 2010, 12, 10348–10355. [Google Scholar] [CrossRef] [PubMed]
- Clairbone, A. Catalase activity. In CRC Handbook of Methods for Oxygen Radicals Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 283–284. [Google Scholar]
- Lee, H.; Lee, M.Y.; Bhang, S.H.; Kim, B.S.; Kim, Y.S.; Ju, J.H.; Kim, K.S.; Hahn, S.K. Hyaluronate-gold nanoparticle/tocilizumab complex for the treatment of rheumatoid rrthritis. ACS Nano 2014, 8, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Qin, H.; Cheng, C.; Xia, Y.; He, C.; Nie, C.; Wang, L.; Zhao, C. Mussel-inspired self-coating at macro-interface with improved biocompatibility and bioactivity via dopamine grafted heparin-like polymers and heparin. J. Mater. Chem. B 2014, 2, 363–375. [Google Scholar] [CrossRef]












| Different Kinds of Water | Preparation Methods | Novel Properties | Representative Reference |
|---|---|---|---|
| Hydrogen-rich water | Adding saturated hydrogen gas in water | Scavenging free radicals | [13] |
| Acidic cosmetic water | Adding acidic compound in water | Scavenging free radicals | [19] |
| Sulfurous water | Adding sulfur compound in water | Scavenging free radicals | [20] |
| Engineered water | Electrospraying water vapor | Inactivate foodborne microorganisms | [42] |
| Magnetic water | Applying magnetic fields on water | Reduced hydrogen bonds (HBs) | [45] |
| Plasmon-activated water/this work | Resonant illumination on gold-supported nanoparticles (AuNPs) in water | Scavenging free radicals, reduced HBs and energetic | [23] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-P.; Liu, Y.-C. Therapeutics for Inflammatory-Related Diseases Based on Plasmon-Activated Water: A Review. Int. J. Mol. Sci. 2018, 19, 1589. https://doi.org/10.3390/ijms19061589
Yang C-P, Liu Y-C. Therapeutics for Inflammatory-Related Diseases Based on Plasmon-Activated Water: A Review. International Journal of Molecular Sciences. 2018; 19(6):1589. https://doi.org/10.3390/ijms19061589
Chicago/Turabian StyleYang, Chih-Ping, and Yu-Chuan Liu. 2018. "Therapeutics for Inflammatory-Related Diseases Based on Plasmon-Activated Water: A Review" International Journal of Molecular Sciences 19, no. 6: 1589. https://doi.org/10.3390/ijms19061589