Osteogenic Differentiation Modulates the Cytokine, Chemokine, and Growth Factor Profile of ASCs and SHED
Abstract
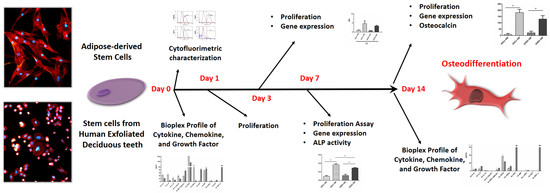
:1. Introduction
2. Results
2.1. ASCs and SHED Displayed Different Cell Morphology and Bimolecular Profiles at First Passage
2.2. Cell Viability
2.3. Early Osteogenic Differentiation of ASCs and SHED
2.4. Late Osteogenic Differentiation of ASCs and SHED
2.5. The Biomolecular Profile of ASCs and SHED Was Modulated upon Culture in OM
3. Discussion
4. Materials and Methods
4.1. Primary Cell Harvest and Culture
4.2. Cell Morphology
4.3. Phenotype of ASCs and SHED
4.4. Detection of Interleukins, Chemokines, and Growth Factors by Bio-Plex System
4.5. Viability Assay
4.6. RNA Extraction and Real-time PCR Analysis
4.7. Alkaline Phosphatase Activity
4.8. Osteocalcin Detection
4.9. Von Kossa Staining
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stem cells |
| ASCs | Adipose-derived stem cells |
| SHED | Stem cells from human exfoliated deciduous teeth |
| DPSCs | Dental pulp stem cells |
| GM | Growth medium |
| OM | Osteogenic medium |
| VEGF | Vascular endothelial growth factor |
| FGFs | Fibroblast growth factors |
| IGF-1 | Insulin growth factor 1 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| GROα | Growth-regulated alpha protein precursor |
| HGF | Hepatocyte growth factor |
| MIF | Macrophage migration inhibitory factor |
| β-NGF | β-Nerve growth factor |
| SCF | Stem cell factor |
| SDF-1α | Stromal cell-derived factor 1-α |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| PBS | Phosphate buffered saline |
| ALP | Alkaline phosphatase |
| OCN | Osteocalcin |
References
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barrena, E.; Rosset, P.; Müller, I.; Giordano, R.; Bunu, C.; Layrolle, P.; Konttinen, Y.T.; Luyten, F.P. Bone regeneration: Stem cell therapies and clinical studies in orthopaedics and traumatology. J. Cell. Mol. Med. 2011, 15, 1266–1286. [Google Scholar] [CrossRef] [PubMed]
- Lichte, P.; Pape, H.C.; Pufe, T.; Kobbe, P.; Fischer, H. Scaffolds for bone healing: Concepts, materials and evidence. Injury 2011, 42, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, H. Biology of bone transplantation. Orthop. Clin. N. Am. 1987, 18, 187–196. [Google Scholar]
- Buser, Z.; Brodke, D.S.; Youssef, J.A.; Meisel, H.-J.; Myhre, S.L.; Hashimoto, R.; Park, J.-B.; Tim Yoon, S.; Wang, J.C. Synthetic bone graft versus autograft or allograft for spinal fusion: A systematic review. J. Neurosurg. Spine 2016, 25, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Khaled, E.G.; Saleh, M.; Hindocha, S.; Griffin, M.; Khan, W.S. Tissue engineering for bone production- stem cells, gene therapy and scaffolds. Open Orthop. J. 2011, 5 (Suppl. 2), 289–295. [Google Scholar] [CrossRef] [PubMed]
- Derubeis, A.R.; Cancedda, R. Bone marrow stromal cells (BMSCs) in bone engineering: Limitations and recent advances. Ann. Biomed. Eng. 2004, 32, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Melo, L.G. Bone Marrow-Derived Mesenchymal Stem Cells: Isolation, Expansion, Characterization, Viral Transduction, and Production of Conditioned Medium. Methods Mol. Biol. 2009, 482, 281–294. [Google Scholar] [PubMed]
- McElreavey, K.D.; Irvine, A.I.; Ennis, K.T.; McLean, W.H. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem. Soc. Trans. 1991, 19, 29S. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Deepe, R.; Hoelscher, G.L.; Ingram, J.A.; Norton, H.J.; Scannell, B.; Loeffler, B.J.; Zinchenko, N.; Hanley, E.N.; Tapp, H. Human adipose-derived mesenchymal stem cells: Direction to a phenotype sharing similarities with the disc, gene expression profiling, and coculture with human annulus cells. Tissue Eng. Part A 2010, 16, 2843–2860. [Google Scholar] [CrossRef] [PubMed]
- In ’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.J.S.; Claas, F.H.J.; Fibbe, W.E.; Kanhai, H.H.H. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Munaron, L.; Carossa, S.; Mussano, F. Overcoming physical constraints in bone engineering: ‘the importance of being vascularized’. J. Biomater. Appl. 2016, 30, 940–951. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Ponnaiyan, D.; Bhat, K.M.; Bhat, G.S. Comparison of Immuno-Phenotypes of Stem Cells from Human Dental Pulp and Periodontal Ligament. Int. J. Immunopathol. Pharmacol. 2012, 25, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; de Alencar, A.H.G.; Kitten, G.T.; Vencio, E.F.; Gava, E. Mesenchymal stem cells in the dental tissues: Perspectives for tissue regeneration. Braz. Dent. J. 2011, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Uzbas, F.; May, I.D.; Parisi, A.M.; Thompson, S.K.; Kaya, A.; Perkins, A.D.; Memili, E. Molecular Physiognomies and Applications of Adipose-Derived Stem Cells. Stem Cell Rev. Rep. 2015, 11, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- De Mendonca Costa, A.; Bueno, D.F.; Martins, M.T.; Kerkis, I.; Kerkis, A.; Fanganiello, R.D.; Cerruti, H.; Alonso, N.; Passos-Bueno, M.R. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J. Craniofac. Surg. 2008, 19, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Makino, Y.; Yamaza, H.; Akiyama, K.; Hoshino, Y.; Song, G.; Kukita, T.; Nonaka, K.; Shi, S.; Yamaza, T. Cryopreserved Dental Pulp Tissues of Exfoliated Deciduous Teeth Is a Feasible Stem Cell Resource for Regenerative Medicine. PLoS ONE 2012, 7, e51777. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker Junior, A.; Gomes Pinheiro, C.C.; Lazzaretti Fernandes, T.; Franco Bueno, D. The use of human dental pulp stem cells for in vivo bone tissue engineering: A systematic review. J. Tissue Eng. 2018, 9, 204173141775276. [Google Scholar] [CrossRef] [PubMed]
- Yorukoglu, A.C.; Kiter, A.E.; Akkaya, S.; Satiroglu-Tufan, N.L.; Tufan, A.C. A Concise Review on the Use of Mesenchymal Stem Cells in Cell Sheet-Based Tissue Engineering with Special Emphasis on Bone Tissue Regeneration. Stem Cells Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Corsalini, M.; Schierano, G.; Pettini, F.; di Venere, D.; Carossa, S. Cytokine, Chemokine, and Growth Factor Profile Characterization of Undifferentiated and Osteoinduced Human Adipose-Derived Stem Cells. Stem Cells Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Shibata, R.; Yamamoto, N.; Nishikawa, M.; Hibi, H.; Tanigawa, T.; Ueda, M.; Murohara, T.; Yamamoto, A. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci. Rep. 2015, 5, 16295. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Alotto, D.; Belisario, D.C.; Casarin, S.; Fumagalli, M.; Cambieri, I.; Piana, R.; Stella, M.; Ferracini, R.; Castagnoli, C. Adipose Derived-Mesenchymal Stem Cells Viability and Differentiating Features for Orthopaedic Reparative Applications: Banking of Adipose Tissue. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.I.; Pereira, H.; Silva-Correia, J.; Van Dijk, C.N.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Current concepts: Tissue engineering and regenerative medicine applications in the ankle joint. J. R. Soc. Interface 2014, 11, 20130784. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Lendeckel, S.; Jödicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.-P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J. Cranio-Maxillofac. Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 2016, 99, 62–68. [Google Scholar] [CrossRef] [PubMed]
- DuPont, N.C.; Wang, K.; Wadhwa, P.D.; Culhane, J.F.; Nelson, E.L. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: Determinations of a panel of nine cytokines in clinical sample culture supernatants. J. Reprod. Immunol. 2005, 66, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Munaron, L.; Petrillo, S.; Erovigni, F.; Carossa, S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 2016, 27, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nishizawa, T.; Hagiya, M.; Seki, T.; Shimonishi, M.; Sugimura, A.; Tashiro, K.; Shimizu, S. Molecular cloning and expression of human hepatocyte growth factor. Nature 1989, 342, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R.; Matsumoto, K.; Nakamura, T.; Orci, L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 1991, 67, 901–908. [Google Scholar] [CrossRef]
- Nakamura, T.; Sakai, K.; Nakamura, T.; Matsumoto, K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J. Gastroenterol. Hepatol. 2011, 26, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.T.; Wang, D.-A. Stromal cell-derived factor-1 (SDF-1): Homing factor for engineered regenerative medicine. Expert Opin. Biol. Ther. 2011, 11, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Dufrane, D. Impact of Age on Human Adipose Stem Cells for Bone Tissue Engineering. Cell Transplant. 2017, 26, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Siegel, K.R.; Clevenger, T.N.; Clegg, D.O.; Proctor, D.A.; Proctor, C.S. Adipose Stem Cells Incorporated in Fibrin Clot Modulate Expression of Growth Factors. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 34, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Murohara, T.; Shintani, S.; Kondo, K. Autologous adipose-derived regenerative cells for therapeutic angiogenesis. Curr. Pharm. Des. 2009, 15, 2784–2790. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Jang, K.A.; Sung, J.H.; Park, J.S.; Kwon, Y.H.; Kim, K.J.; Kim, W.S. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol. Surg. 2008, 34, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.; Perlman, H. Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 1997, 7, 597–602. [Google Scholar] [CrossRef]
- Buttitta, L.A.; Edgar, B.A. Mechanisms controlling cell cycle exit upon terminal differentiation. Curr. Opin. Cell Biol. 2007, 19, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.; Alam, P.; Pacelli, S.; Chakravarti, A.R.; Ahmed, R.P.H.; Paul, A. Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater. 2017, 69, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Denkovskij, J.; Bagdonas, E.; Kusleviciute, I.; Mackiewicz, Z.; Unguryte, A.; Porvaneckas, N.; Fleury, S.; Venalis, A.; Jorgensen, C.; Bernotiene, E. Paracrine Potential of the Human Adipose Tissue-Derived Stem Cells to Modulate Balance between Matrix Metalloproteinases and Their Inhibitors in the Osteoarthritic Cartilage In Vitro. Stem Cells Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Ishigami, M.; Matsubara, K.; Kondo, M.; Wakayama, H.; Goto, H.; Ueda, M.; Yamamoto, A. Multifaceted therapeutic benefits of factors derived from stem cells from human exfoliated deciduous teeth for acute liver failure in rats. J. Tissue Eng. Regen. Med. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, H.; Hashimoto, N.; Matsushita, Y.; Matsubara, K.; Yamamoto, N.; Hasegawa, Y.; Ueda, M.; Yamamoto, A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy 2015, 17, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Hristov, M.; Zernecke, A.; Liehn, E.A.; Weber, C. Regulation of endothelial progenitor cell homing after arterial injury. Thromb. Haemost. 2007, 98, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Verga Falzacappa, E.; Scopece, P.; Munaron, L.; Rivolo, P.; Mandracci, P.; Benedetti, A.; Carossa, S.; Patelli, A. In vitro characterization of two different atmospheric plasma jet chemical functionalizations of titanium surfaces. Appl. Surf. Sci. 2017, 409, 314–324. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Rivolo, P.; Mandracci, P.; Munaron, L.; Faga, M.G.; Carossa, S. Role of surface finishing on the in vitro biological properties of a silicon nitride–titanium nitride (Si3N4–TiN) composite. J. Mater. Sci. 2017, 52, 467–477. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Mandracci, P.; Mussano, F.; Abundo, R.; Fiorellini, J. Morphometric Changes Induced by Cold Argon Plasma Treatment on Osteoblasts Grown on Different Dental Implant Surfaces. Int. J. Periodontics Restor. Dent. 2017, 37, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Grolez, G.P.; Camillo, C.; Bernardini, M.; Bokhobza, A.; Richard, E.; Scianna, M.; Lemonnier, L.; Valdembri, D.; Munaron, L.; et al. TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1. J. Cell Biol. 2017, 216, 2107–2130. [Google Scholar] [CrossRef] [PubMed]
- Fiorio Pla, A.; Genova, T.; Pupo, E.; Tomatis, C.; Genazzani, A.; Zaninetti, R.; Munaron, L. Multiple Roles of Protein Kinase A in Arachidonic Acid-Mediated Ca2+ Entry and Tumor-Derived Human Endothelial Cell Migration. Mol. Cancer Res. 2010, 8, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Mussano, F.; Franchi, S.; Panerai, A.E.; Bussolati, G.; Carossa, S.; Bartorelli, A.; Bussolati, B. Biological components in a standardized derivative of bovine colostrum. J. Dairy Sci. 2013, 96, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Faga, M.G.; Mussano, F.; Catalano, F.; Tolosano, E.; Carossa, S.; Altruda, F.; Martra, G. Alumina-zirconia composites functionalized with laminin-1 and laminin-5 for dentistry: Effect of protein adsorption on cellular response. Colloids Surf. B Biointerfaces 2014, 114, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Munaron, L.; Genova, T.; Avanzato, D.; Antoniotti, S.; Fiorio Pla, A. Targeting calcium channels to block tumor vascularization. Recent Pat. Anticancer Drug Discov. 2013, 8, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Laurenti, M.; Zicola, E.; Munaron, L.; Rivolo, P.; Mandracci, P.C.S. Early response of fibroblasts and epithelial cells to pink-shaded anodized dental implant abutments: An in vitro study. Int. J. Oral Maxillofac. Implants 2018, in press. [Google Scholar]
- Petrillo, S.; Chiabrando, D.; Genova, T.; Fiorito, V.; Ingoglia, G.; Vinchi, F.; Mussano, F.; Carossa, S.; Silengo, L.; Altruda, F.; et al. Heme accumulation in endothelial cells impairs angiogenesis by triggering paraptosis. Cell Death Differ. 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Schierano, G.; Mussano, F.; Faga, M.G.; Menicucci, G.; Manzella, C.; Sabione, C.; Genova, T.; von Degerfeld, M.M.; Peirone, B.; Cassenti, A.; et al. An Alumina Toughened Zirconia Composite for Dental Implant Application: In Vivo Animal Results. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Belisario, D.C.; Compagno, M.; Verderio, L.; Sighinolfi, A.; Mussano, F.; Genova, T.; Veneziano, F.; Pertici, G.; Perale, G.; et al. Adipose-derived stromal vascular fraction/xenohybrid bone scaffold: An alternative source for bone regeneration. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef]
- Yang, R.; Davies, C.M.; Archer, C.W.; Richards, R.G. Immunohistochemistry of matrix markers in Technovit 9100 New-embedded undecalcified bone sections. Eur. Cells Mater. 2003, 6, 57–71. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of Argon Affects the Earliest Biological Response of Different Implant Surfaces. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Fiorio Pla, A.; Brossa, A.; Bernardini, M.; Genova, T.; Grolez, G.; Villers, A.; Leroy, X.; Prevarskaya, N.; Gkika, D.; Bussolati, B. Differential sensitivity of prostate tumor derived endothelial cells to sorafenib and sunitinib. BMC Cancer 2014, 14, 939. [Google Scholar] [CrossRef] [PubMed]






| Biomolecule | Baseline | |
|---|---|---|
| ASCs | SHED | |
| Hu IL-6 | 44.40 | 22.12 |
| Hu IL-8 | 46.13 | 7.76 |
| Hu IL-10 | 21.78 | 10.55 |
| Hu IL-12 | 36.76 | 19.72 |
| Hu MCP-1 | 35.82 | 73.40 |
| Hu VEGF | 2867.41 | 786.37 |
| Hu GROα | 161.90 | 4.90 |
| Hu HGF | 2.78 | 81.67 |
| Hu MIF | 72.90 | 87.28 |
| Hu β-NGF | 1.33 | 5.68 |
| Hu SCF | 0.01 | 9.51 |
| Hu SDF-1α | 0.01 | 233.43 |
| Biomolecule | 14 Days GM | 14 Days OM | ||
|---|---|---|---|---|
| ASCs | SHED | ASCs | SHED | |
| Hu IL-6 | 173.07 | 125.29 | 3.02 | 5.04 |
| Hu IL-8 | 108.70 | 192.72 | 4.95 | 11.10 |
| Hu IL-10 | 7.24 | 6.95 | 0.62 | 0.39 |
| Hu IL-12 | 12.41 | 12.73 | 0.01 | 0.01 |
| Hu MCP-1 | 65.23 | 76.71 | 2.87 | 6.80 |
| Hu VEGF | 426.58 | 486.12 | 37.25 | 22.67 |
| Hu GROα | 98.51 | 25.85 | 5.99 | 11.03 |
| Hu HGF | 1.43 | 83.51 | 0.10 | 79.66 |
| Hu MIF | 8.54 | 176.84 | 0.10 | 0.00 |
| Hu β-NGF | 0.10 | 8.87 | 0.17 | 1.45 |
| Hu SCF | 0.01 | 8.02 | 0.01 | 0.10 |
| Hu SDF-1α | 0.01 | 373.04 | 0.01 | 183.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussano, F.; Genova, T.; Petrillo, S.; Roato, I.; Ferracini, R.; Munaron, L. Osteogenic Differentiation Modulates the Cytokine, Chemokine, and Growth Factor Profile of ASCs and SHED. Int. J. Mol. Sci. 2018, 19, 1454. https://doi.org/10.3390/ijms19051454
Mussano F, Genova T, Petrillo S, Roato I, Ferracini R, Munaron L. Osteogenic Differentiation Modulates the Cytokine, Chemokine, and Growth Factor Profile of ASCs and SHED. International Journal of Molecular Sciences. 2018; 19(5):1454. https://doi.org/10.3390/ijms19051454
Chicago/Turabian StyleMussano, Federico, Tullio Genova, Sara Petrillo, Ilaria Roato, Riccardo Ferracini, and Luca Munaron. 2018. "Osteogenic Differentiation Modulates the Cytokine, Chemokine, and Growth Factor Profile of ASCs and SHED" International Journal of Molecular Sciences 19, no. 5: 1454. https://doi.org/10.3390/ijms19051454