Role of Macrophages in Brain Tumor Growth and Progression
Abstract
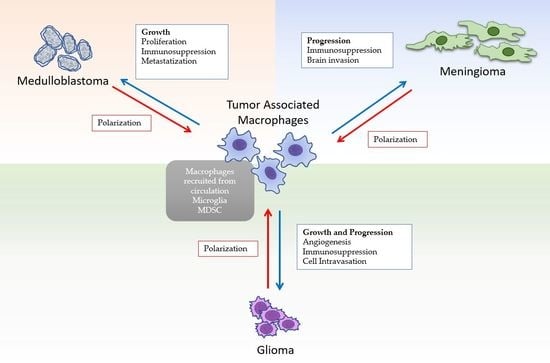
:1. Introduction
2. Microglia and Macrophages
3. Gliomas
3.1. Tumor Associated Macrophages and “Malignant” Macrophages in Gliomas
3.2. TAMs and Angiogenesis
3.3. TAMs Immunosoppressive Activity
4. Meningiomas
5. Medulloblastoma
6. Discussion
Conflicts of Interest
References
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS immune privilege: Hiding in plain sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.W.; Gu, C. The molecular constituents of the blood-brain barrier. Trends Neurosci. 2015, 38, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Ransohoff, R.M. Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. 2012, 33, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012, 12, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018, 20, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Corthay, A. Does the immune system naturally protect against cancer? Front. Immunol. 2014, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Magaña-Maldonado, R.; Chávez-Cortez, E.G.; Olascoaga-Arellano, N.K.; López-Mejía, M.; Maldonado-Leal, F.M.; Sotelo, J.; Pineda, B. Immunological Evasion in Glioblastoma. BioMed Res. Int. 2016, 2016, 7487313. [Google Scholar] [CrossRef] [PubMed]
- Rolle, C.E.; Sengupta, S.; Lesniak, M.S. Mechanisms of immune evasion by gliomas. Adv. Exp. Med. Biol. 2012, 746, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Nardin, A.; Abastado, J.P. Macrophages and cancer. Front. Biosci. 2008, 13, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Dehne, N.; Mora, J.; Namgaladze, D.; Weigert, A.; Brüne, B. Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr. Opin. Pharmacol. 2017, 35, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Brouland, J.P.; Hottinger, A.F. Revised WHO classification 2016 of gliomas: What’s new? Rev. Med. Suisse 2017, 13, 1805–1809. [Google Scholar] [PubMed]
- Wesseling, P.; Capper, D. WHO 2016 Classification of Gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Louis, N.D.; Ohgaki, H.; Cavenee, W.K.; Ellison, D.W.; Figarella-Branger, D.; Perry, A.; Reifenberger, G.; von Deimling, A. WHO Classification of Tumours of the Central Nervous System, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2016; ISBN 978-92-832-4492-9. [Google Scholar]
- Ginhoux, F.; Merad, M. Microglia arise from extra-embryonic yolk sac primitive progenitors. Med. Sci. 2011, 27, 719–724. [Google Scholar] [CrossRef]
- Heusinkveld, M.; van der Burg, S.H. Identification and manipulation of tumor-associated macrophages in human cancers. J. Transl. Med. 2011, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Pey, P.; Pearce, R.K.; Kalaitzakis, M.E.; Griffin, W.S.; Gentleman, S.M. Phenotypic profile of alternative activation marker CD163 is different in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. Commun. 2014, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Fabriek, B.O.; Van Haastert, E.S.; Galea, I.; Polfliet, M.M.; Döpp, E.D.; Van Den Heuvel, M.M.; Van Den Berg, T.K.; De Groot, C.J.; Van Der Valk, P.; Dijkstra, C.D. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia 2005, 51, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Borda, J.T.; Alvarez, X.; Mohan, M.; Hasegawa, A.; Bernardino, A.; Jean, S.; Aye, P.; Lackner, A.A. CD163, a marker of perivascular macrophages, is up-regulated by microglia in simian immunodeficiency virus encephalitis after haptoglobin-hemoglobin complex stimulation and is suggestive of breakdown of the blood-brain barrier. Am. J. Pathol. 2008, 172, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.E. Histology for Pathologists, 3rd ed.; Lippincott: Philadelphia, PA, USA, 2007; pp. 298–299. ISBN 978-1451113037. [Google Scholar]
- Hulette, C.M. Microglioma, a histiocytic neoplasm of the central nervous system. Mod. Pathol. 1996, 9, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.; Ohsawa, K.; Buckland, M.E.; Kesavadas, C.; Ratheesan, K.; Kusumakumary, P.; Burger, P.C.; Kohsaka, S.; Graeber, M.B. Microglioma in a child—A further case in support of the microglioma entity and distinction from histiocytic sarcoma. Clin. Neuropathol. 2016, 35, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Strojnik, T.; Kavalar, R.; Zajc, I.; Diamandis, E.P.; Oikonomopoulou, K.; Lah, T.T. Prognostic impact of CD68 and kallikrein 6 in human glioma. Anticancer Res. 2009, 29, 3269–3279. [Google Scholar] [PubMed]
- Leenstra, S.; Das, P.K.; Troost, D.; de Boer, O.J.; Bosch, D.A. Human malignant astrocytes express macrophage phenotype. J. Neuroimmunol. 1995, 56, 17–25. [Google Scholar] [CrossRef]
- Huysentruyt, L.C.; Akgoc, Z.; Seyfried, T.N. Hypothesis: Are neoplastic macrophages/microglia present in glioblastoma multiforme? ASN Neuro 2011, 3, e00064. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kubota, T.; Kitai, R.; Nakagawa, R.; Hashimoto, N. CD98 immunoreactivity in multinucleated giant cells of glioblastomas: An immunohistochemical double labeling study. Neuropathology 2008, 28, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Megova, M.; Drabek, J.; Koudelakova, V.; Trojanec, R.; Kalita, O.; Hajduch, M. Isocitrate dehydrogenase 1 and 2 mutations in gliomas. J. Neurosci. Res. 2014, 92, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Waitkus, M.S.; Diplas, B.H.; Yan, H. Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol. 2016, 18, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Herriot, K. Practical Surgical Pathology; Amerian Society for Clinical Pathology: Hong Kong, China, 2013; p. 424. ISBN 978-089189-5886. [Google Scholar]
- Morantz, R.A.; Wood, G.W.; Foster, M.; Clark, M.; Gollahon, K. Macrophages in experimental and human brain tumors. Part 2: Studies of the macrophage content of human brain tumors. J. Neurosurg. 1979, 50, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.L.; Hughes, J.T.; Esiri, M.M.; Coakham, H.B.; Brownell, D.B. Immunohistological study of mononuclear cell infiltrate in malignant gliomas. Acta Neuropathol. 1987, 74, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Roggendorf, W.; Strupp, S.; Paulus, W. Distribution and characterization of microglia/macrophages in human braintumors. Acta Neuropathol. 1996, 92, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, C.; Signorelli, F.; Vismara, M.F.; Zeppa, P.; Camastra, C.; Barni, T.; Donato, G.; Di Vito, A. A reappraisal of macrophage polarization in glioblastoma: Histopathological and immunohistochemical findings and review of the literature. Pathol. Res. Pract. 2016, 212, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Tellier, C.; Feron, O. Cycling hypoxia: A key feature of the tumor microenvironment. Biochim. Biophys. Acta 2016, 1866, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.C.; Scheithauer, B.W. Neuropathology; Amirsys Publishing: Salt Lake City, UT, USA, 2012; pp. 17–30. ISBN 978-1-931884-58-7. [Google Scholar]
- Evans, S.M.; Judy, K.D.; Dunphy, I.; Jenkins, W.T.; Hwang, W.T.; Nelson, P.T.; Lustig, R.A.; Jenkins, K.; Magarelli, D.P.; Hahnm, S.M.; et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin. Cancer Res. 2004, 10, 8177–8184. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Terasaka, S.; Shiga, T.; Hattori, N.; Magota, K.; Kobayashi, H.; Yamaguchi, S.; Houkin, K.; Tanaka, S.; Kuge, Y.; et al. 18F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Cramer, T.; Yamanishi, Y.; Clausen, B.E.; Förster, I.; Pawlinski, R.; Mackman, N.; Haase, V.H.; Jaenisch, R.; Corr, M.; Nizet, V.; et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 2003, 112, 645–657. [Google Scholar] [CrossRef]
- Leblond, M.M.; Gérault, A.N.; Corroyer-Dulmont, A.; MacKenzie, E.T.; Petit, E.; Bernaudin, M.; Valable, S. Hypoxia induces macrophage polarization and re-education toward an M2 phenotype in U87 and U251 glioblastoma models. Oncoimmunology 2015, 5, e1056442. [Google Scholar] [CrossRef] [PubMed]
- Laoui, D.; Van Overmeire, E.; Di Conza, G.; Aldeni, C.; Keirsse, J.; Morias, Y.; Movahedi, K.; Houbracken, I.; Schouppe, E.; Elkrim, Y.; et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014, 74, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Henze, A.T.; Mazzone, M. The impact of hypoxia on tumor-associated macrophages. J. Clin. Investig. 2016, 126, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Lisi, L.; Stigliano, E.; Lauriola, L.; Navarra, P.; Dello Russo, G. Proinflammatory-activated glioma cells induce a switch in microglial polarization and activation status, from a predominant M2b phenotype to a mixture of M1 and M2a/B polarized cells. ASN Neuro 2014, 6, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, S.; Müller, A.; Turkowski, K.; Radev, Y.T.; Rot, S.; Schmidt, C.; Bungert, A.D.; Acker, G.; Schorr, A.; Hippe, A.; et al. Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol. 2016, 131, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Zhang, I.Y.; Liang, J.; Wang, H.; Ouyang, M.; Wu, S.; da Fonseca, A.C.C.; Weng, L.; Yamamoto, Y.; et al. RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res. 2014, 74, 7285–7297. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, N.; Ferrara, N.; Vacher, J.; Gaedicke, S.; Niedermann, G.; Weyerbrock, A.; Doostkam, S.; Schaefer, H.E.; Plate, K.H.; Machein, M.R. Decrease of VEGF-A in myeloid cells attenuates glioma progression and prolongs survival in an experimental glioma model. Neuro Oncol. 2016, 18, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017; ISBN 978-0-9944381-2-6. [Google Scholar]
- Tosoni, A.; Franceschi, E.; Poggi, R.; Brandes, A.A. Relapsed Glioblastoma: Treatment Strategies for Initial and Subsequent Recurrences. Curr. Treat. Opt. Oncol. 2016, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Turkowski, K.; Brandenburg, S.; Mueller, A.; Kremenetskaia, I.; Bungert, A.D.; Blank, A.; Felsenstein, M.; Vajkoczy, P. VEGF as a modulator of the innate immune response in glioblastoma. Glia 2018, 66, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Heddleston, J.M.; Li, Z.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 2009, 8, 3274–3284. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.F.; Yao, X.H.; Jiang, J.Y.; Zhao, L.T.; Yu, S.C.; Jiang, T.; Lin, M.C.; Chen, J.H.; Wang, B.; Zhang, R.; et al. The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signaling. J. Pathol. 2011, 224, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Hattermann, K.; Sebens, S.; Helm, O.; Schmitt, A.D.; Mentlein, R.; Mehdorn, H.M.; Held-Feindt, J. Chemokine expression profile of freshly isolated human glioblastoma-associated macrophages/microglia. Oncol. Rep. 2014, 32, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kioi, M.; Vogel, H.; Schultz, G.; Hoffman, R.M.; Harsh, G.R.; Brown, J.M. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Investig. 2010, 120, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Stafford, J.H.; Liu, S.C.; Chernikova, S.B.; Merchant, M.; Recht, L.; Martin Brown, J. SDF-1 Blockade Enhances Anti-VEGF Therapy of Glioblastoma and Can Be Monitored by MRI. Neoplasia 2017, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; De Palma, M.; Naldini, L. Tie2-expressing monocytes and tumor angiogenesis: Regulation by hypoxia and angiopoietin-2. Cancer Res. 2007, 67, 8429–8432. [Google Scholar] [CrossRef] [PubMed]
- Venneri, M.A.; De Palma, M.; Ponzoni, M.; Pucci, F.; Scielzo, C.; Zonari, E.; Mazzieri, R.; Doglioni, C.; Naldini, L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 2007, 109, 5276–5285. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Lu, K.V.; Petritsch, C.; Liu, P.; Ganss, R.; Passegué, E.; Song, H.; Vandenberg, S.; Johnson, R.S.; Werb, Z.; et al. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 2008, 13, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, L.; Okcu, M.F.; Wang, T.; Cao, Y.; Renfro-Spelman, A.; Aldape, K.D.; Gilbert, M.R.; Bondy, M. Glutathione S-transferase polymorphisms are associated with survival in anaplastic glioma patients. Cancer 2010, 116, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Liebelt, B.D.; Shingu, T.; Zhou, X.; Ren, J.; Shin, S.A.; Hu, J. Glioma Stem Cells: Signaling, Microenvironment, and Therapy. Stem Cells Int. 2016, 2016, 7849890. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kros, J.M.; Cheng, C.; Mustafa, D. The contribution of tumor-associated macrophages in glioma neo-angiogenesis and implications for anti-angiogenic strategies. Neuro Oncol. 2017, 19, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A. The multifaceted profile of activated microglia. Mol. Neurobiol. 2009, 40, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Follesa, P.; Citraro, R.; Camastra, C.; Donato, A.; Isola, D.; Constanti, A.; De Sarro, G.; Donato, G. The mTOR signaling pathway and neuronal stem/progenitor cell proliferation in the hippocampus are altered during the development of absence epilepsy in a genetic animal model. Neurol. Sci. 2014, 35, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Shaw, G.; Sharma, V.P.; Yang, C.; McGowan, E.; Dickson, D.W. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J. Histochem. Cytochem. 2007, 55, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Gabrusiewicz, K.; Heimberger, A. The controversial role of microglia in malignant gliomas. Clin. Dev. Immunol. 2013, 2013, 285246. [Google Scholar] [CrossRef] [PubMed]
- Wurdinger, T.; Deumelandt, K.; van der Vliet, H.J.; Wesseling, P.; de Gruijl, T.D. Mechanisms of intimate and long-distance cross-talk between glioma and myeloid cells: How to break a vicious cycle. Biochim. Biophys. Acta 2014, 1846, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, C.; Scali, E.; Camastra, C.; Presta, I.; Zeppa, P.; Barni, T.; Donato, G.; Bottoni, U.; Di Vito, A. Innate immunity in cutaneous melanoma. Clin. Exp. Dermatol. 2017, 42, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Modolell, M.; Corraliza, I.M.; Link, F.; Soler, G.; Eichmann, K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur. J. Immunol. 1995, 25, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Renne, M.; Conforti, F.; Camastra, C.; Donato, A.; Donato, G. Macrophage Activation and Patterns of Inflammation in Obese and Non-Obese Women with Breast Carcinoma. Eur. J. Inflamm. 2014, 12, 197–200. [Google Scholar] [CrossRef]
- Di Vito, A.; Santise, G.; Mignogna, C.; Chiefari, E.; Cardillo, G.; Presta, I.; Arturi, F.; Malara, N.; Brunetti, F.; Donato, A.; et al. Innate immunity in cardiac myxomas and its pathological and clinical correlations. Innate Immun. 2018, 24, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Scali, E.; Mignogna, C.; Di Vito, A.; Presta, I.; Camastra, C.; Donato, G.; Bottoni, U. Inflammation and macrophage polarization in cutaneous melanoma: Histopathological and immunohistochemical study. Int. J. Immunopathol. Pharmacol. 2016, 29, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Presta, I.; Guadagno, E.; Di Vito, A.; Malara, N.; Mignogna, C.; Maisano, D.; Donato, A.; Cardillo, G.; Del Basso De Caro, M.L.; Donato, G. Innate immunity may play a role in growth and relapse of chordoid meningioma. Int. J. Immunopathol. Pharmacol. 2017, 30, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Kitamura, Y.; Takahashi, H.; Tooyama, I.; Kimura, H.; Gebicke-Haerter, P.J.; Nomura, Y.; Taniguchi, T. Interferon-gamma plus lipopolysaccharide induction of delayed neuronal apoptosis in rat hippocampus. Neurochem. Int. 1999, 34, 91–99. [Google Scholar] [CrossRef]
- Zhang, Z.; Jyoti, A.; Balakrishnan, B.; Williams, M.; Singh, S.; Chugani, D.C.; Kannan, S. Trajectory of inflammatory and microglial activation markers in the postnatal rabbit brain following intrauterine endotoxin exposure. Neurobiol. Dis. 2017, 111, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Huang, Z.; Sun, X.; Zhu, X.; Zhou, L.; Li, M.; Cheng, B.; Liu, X.; He, C. Microglia Polarization with M1/M2 Phenotype Changes in rd1 Mouse Model of Retinal Degeneration. Front. Neuroanat. 2017, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kros, J.M.; van der Weiden, M.; Zheng, P.; Cheng, C.; Mustafa, D.A. Expression site of P2RY12 in residential microglial cells in astrocytomas correlates with M1 and M2 marker expression and tumor grade. Acta Neuropathol. Commun. 2017, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.F.; Yang, D.; Suki, D.; Aldape, K.; Grimm, E.; Heimberger, A.B. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006, 8, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Wei, J.; Kong, L.Y.; Wang, Y.; Priebe, W.; Qiao, W.; Sawaya, R.; Heimberger, A.B. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010, 12, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Hishii, M.; Nitta, T.; Ishida, H.; Ebato, M.; Kurosu, A.; Yagita, H.; Sato, K.; Okumura, K. Human glioma-derived interleukin-10 inhibits antitumor immune responses in vitro. Neurosurgery 1995, 37, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Uhl, M.; Ma, J.Y.; Janssen, L.; Sriram, V.; Aulwurm, S.; Kerr, I.; Lam, A.; Webb, H.K.; Kapoun, A.M.; et al. Inhibiting TGF-𝛽 signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol. 2007, 9, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sarkar, S.; Cua, R.; Zhou, Y.; Hader, W.; Yong, V.W. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis 2012, 33, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Brady, W.; Urnes, M.; Grosmaire, L.S.; Damle, N.K.; Ledbetter, J.A. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991, 174, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Fecci, P.E.; Ochiai, H.; Mitchell, D.A.; Grossi, P.M.; Sweeney, A.E.; Archer, G.E.; Cummings, T.; Allison, J.P.; Bigner, D.D.; Sampson, J.H. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin. Cancer Res. 2007, 13, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, H.; Gu, L.; Ye, B.; Jian, Z.; Stary, C.; Xiong, X. Advances in Immunotherapy for Glioblastoma Multiforme. J. Immunol. Res. 2017, 2017, 3597613. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.V.; Cseh, O.; Aman, A.; Weiss, S.; Luchman, H.A. The JAK2/STAT3 inhibitor pacritinib effectively inhibits patient-derived GBM brain tumor initiating cells in vitro and when used in combination with temozolomide increases survival in an orthotopic xenograft model. PLoS ONE 2017, 12, e0189670. [Google Scholar] [CrossRef] [PubMed]
- Badie, B.; Schartner, J.; Prabakaran, S.; Paul, J.; Vorpahl, J. Expression of Fas ligand by microglia: Possible role in glioma immune evasion. J. Neuroimmunol. 2001, 120, 19–24. [Google Scholar] [CrossRef]
- Kmiecik, J.; Poli, A.; Brons, N.H.; Waha, A.; Eide, G.E.; Enger, P.Ø.; Zimmer, J.; Chekenya, M. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol. 2013, 264, 71–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, I.; Tihan, T.; Han, S.J.; Wrensch, M.R.; Wiencke, J.; Sughrue, M.E.; Parsa, A.T. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J. Clin. Neurosci. 2010, 17, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Mangani, D.; Weller, M.; Roth, P. The network of immunosuppressive pathways in glioblastoma. Biochem. Pharmacol. 2017, 130, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. The 2016 WHO Classification of Tumours of the Central Nervous System: The Major Points of Revision. Neurol. Med. Chir. 2017, 57, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ohba, S.; Hirose, Y. Biological Significance of Mutant Isocitrate Dehydrogenase 1 and 2 in Gliomagenesis. Neurol. Med. Chir. 2016, 56, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Donato, G.; Iofrida, G.; Lavano, A.; Volpentesta, G.; Signorelli, F.; Pallante, P.L.; Berlingieri, M.T.; Pierantoni, M.G.; Palmieri, D.; Conforti, F.; et al. Analysis of UbcH10 expression represents a useful tool for the diagnosis and therapy of astrocytic tumors. Clin. Neuropathol. 2008, 27, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Donato, G.; Martinez Hoyos, J.; Amorosi, A.; Maltese, L.; Lavano, A.; Volpentesta, G.; Signorelli, F.; Pentimalli, F.; Pallante, P.; Ferraro, G.; et al. High mobility group A1 expression correlates with the histological grade of human glial tumors. Oncol. Rep. 2004, 11, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Pagès, F.; Marincola, F.M.; Thurin, M.; Trinchieri, G.; Fox, B.A.; Gajewski, T.F.; Ascierto, P.A. The immune score as a new possible approach for the classification of cancer. J. Transl. Med. 2012, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, A.; Louis, D.N.; Budka, H.; von Deimling, A.; Sahm, F.; Rushing, E.J.; Mawrin, C.; Claus, E.B.; Loeffler, J.; Sadetzki, S. Meningiomas. In WHO Classification of Tumours of the Central Nervous System, 4th ed.; Louis, D.N., Ohgaki, H., Wiestler, O.D., Cavenee, W.K., Eds.; Agency for Research on Cancer: Lyon, France, 2016; ISBN 978-92-832-4492-9. [Google Scholar]
- Marosi, C.; Preusser, M. Milestones of the last 10 years: CNS cancer. Memo 2017, 10, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Preusser, M.; Brastianos, P.K.; Mawrin, C. Advances in meningioma genetics: Novel therapeutic opportunities. Nat. Rev. Neurol. 2018, 14, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017, 18, 682–694. [Google Scholar] [CrossRef]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avşar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Strik, H.M.; Stoll, M.; Meyermann, R. Immune cell infiltration of intrinsic and metastatic intracranial tumours. Anticancer Res. 2004, 24, 37–42. [Google Scholar] [PubMed]
- Rossi, M.L.; Cruz Sanchez, F.; Hughes, J.T.; Esiri, M.M.; Coakham, H.B. Immunocytochemical study of the cellular immune response in meningiomas. J. Clin. Pathol. 1988, 41, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Domingues, P.; González-Tablas, M.; Otero, Á.; Pascual, D.; Miranda, D.; Ruiz, L.; Sousa, P.; Ciudad, J.; Gonçalves, J.M.; Lopes, M.C.; et al. Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav. Immun. 2016, 53, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Grund, S.; Schittenhelm, J.; Roser, F.; Tatagiba, M.; Mawrin, C.; Kim, Y.J.; Bornemann, A. The microglial/macrophagic response at the tumour-brain border of invasive meningiomas. Neuropathol. Appl. Neurobiol. 2009, 35, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Domingues, P.H.; Teodósio, C.; Ortiz, J.; Sousa, P.; Otero, A.; Maillo, A.; Bárcena, P.; García-Macias, M.C.; Lopes, M.C.; de Oliveira, C.; et al. Immunophenotypic identification and characterization of tumor cells and infiltrating cell populations in meningiomas. Am. J. Pathol. 2012, 181, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Fukami, T.; Nitta, N.; Iwai, M.; Yoshida, K.; Kagotani, A.; Nozaki, K.; Okabe, H. Xanthomatous meningioma: A case report with review of the literature. Int. J. Clin. Exp. Pathol. 2013, 6, 2242–2246. [Google Scholar] [PubMed]
- Shabo, I.; Midtbö, K.; Andersson, H.; Åkerlund, E.; Olsson, H.; Wegman, P.; Gunnarsson, C.; Lindström, A. Macrophage traits in cancer cells are induced by macrophage-cancer cell fusion and cannot be explained by cellular interaction. BMC Cancer 2015, 15, 922. [Google Scholar] [CrossRef] [PubMed]
- Kanno, H.; Nishihara, H.; Wang, L.; Yuzawa, S.; Kobayashi, H.; Tsuda, M.; Kimura, T.; Tanino, M.; Terasaka, S.; Tanaka, S. Expression of CD163 prevents apoptosis through the production of granulocyte colony-stimulating factor in meningioma. Neuro Oncol. 2013, 15, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Di Ieva, A.; Laiq, S.; Nejad, R.; Schmitz, E.M.; Fathalla, H.; Karamchandani, J.; Munoz, D.G.; Cusimano, M.D. Chordoid meningiomas: Incidence and clinicopathological features of a case series over 18 years. Neuropathology 2015, 35, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Donato, G.; Ferraro, G.; Signorelli, F.; Iofrida, G.; Lavano, A.; Amorosi, A.; Maltese, L.; Perrotta, I.; Tripepi, S.; Pardatscher, K.; et al. Chordoid meningioma: Case report and literature review. Ultrastruct. Pathol. 2006, 30, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.W.; Ebehart, C.G.; Pietsch, T.; Pfister, S. Medulloblastoma. In WHO Classification of Tumours of the Central Nervous System, 4th ed.; Louis, D.N., Ohgaki, H., Wiestler, O.D., Cavenee, W.K., Eds.; Agency for Research on Cancer: Lyon, France, 2016; ISBN 978-92-832-4492-9. [Google Scholar]
- Northcott, P.A.; Jones, D.T.; Kool, M.; Robinson, G.W.; Gilbertson, R.J.; Cho, Y.J.; Pomeroy, S.L.; Korshunov, A.; Lichter, P.; Taylor, M.D.; et al. Medulloblastomics: The end of the beginning. Nat. Rev. Cancer 2012, 12, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smoll, N.R. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs). Cancer 2012, 118, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.M.; Ghali, M.G.; North, R.Y.; Boghani, Z.; Hansen, D.; Lam, S. Modern management of medulloblastoma: Molecular classification, outcomes, and the role of surgery. Surg. Neurol. Int. 2016, 7, S1135–S1141. [Google Scholar] [CrossRef] [PubMed]
- Margol, A.S.; Robison, N.J.; Gnanachandran, J.; Hung, L.T.; Kennedy, R.J.; Vali, M.; Dhall, G.; Finlay, J.L.; Erdreich-Epstein, A.; Krieger, M.D.; et al. Tumor-associated macrophages in SHH subgroup of medulloblastomas. Clin. Cancer Res. 2015, 21, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Y.; Elghetany, M.T.; Shen, J.; Man, T.K.; Li, X.; Chintagumpala, M.; Su, J.M.; Dauser, R.; Whitehead, W.; Adesina, A.M.; et al. Therapeutic implications of CD1d expression and tumorinfiltrating macrophages in pediatric medulloblastomas. J. Neurooncol. 2014, 120, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Garzia, L.; Kijima, N.; Morrissy, A.S.; De Antonellis, P.; Guerreiro-Stucklin, A.; Holgado, B.L.; Wu, X.; Wang, X.; Parsons, M.; Zayne, K.; et al. A Hematogenous Route for Medulloblastoma Leptomeningeal Metastases. Cell 2018, 172, 1050–1062.e14. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef] [PubMed]
- Schönsteiner, S.S.; Bommer, M.; Haenle, M.M.; Klaus, B.; Scheuerle, A.; Schmid, M.; Mayer-Steinacker, R. Rare phenomenon: Liver metastases from glioblastoma multiforme. J. Clin. Oncol. 2011, 29, e668–e671. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Shirayama, R.; Morita, H.; Kusuhara, K. Pulmonary and pleural metastasis of intracranial anaplastic meningioma in a 3-year-old boy: A case report. Mol. Clin. Oncol. 2017, 7, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Roberts, G.J.; Lucas, V.S.; Barrett, A.W.; Harkness, W. Metastatic infiltration of the dental pulp by medulloblastoma. J. Oral Pathol. Med. 2002, 31, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Linde, N.; Casanova-Acebes, M.; Sosa, M.S.; Mortha, A.; Rahman, A.; Farias, E.; Harper, K.; Tardio, E.; Reyes Torres, I.; Jones, J.; et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 2018, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Che, D.; Yang, F.; Chi, C.; Meng, H.; Shen, J.; Qi, L.; Liu, F.; Lv, L.; Li, Y.; et al. Tumor-associated macrophages promote tumor metastasis via the TGF-β/SOX9 axis in non-small cell lung cancer. Oncotarget 2017, 8, 99801–99815. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.M.; et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Cui, Y.; Jiang, H.; Sui, D.; Wang, Y.; Jiang, Z.; Zhao, J.; Lin, S. Circulating tumor cell is a common property of brain glioma and promotes the monitoring system. Oncotarget 2016, 7, 71330–71340. [Google Scholar] [CrossRef] [PubMed]
- Malara, N.; Guzzi, G.; Mignogna, C.; Trunzo, V.; Camastra, C.; Della Torre, A.; Di Vito, A.; Lavecchia, A.M.; Gliozzi, M.; Ceccotti, C.; et al. Non-invasive real-time biopsy of intracranial lesions using short time expanded circulating tumor cells on glass slide: Report of two cases. BMC Neurol. 2016, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.P.; Nahed, B.V.; Madden, M.W.; Oliveira, S.M.; Springer, S.; Bhere, D.; Chi, A.S.; Wakimoto, H.; Rothenberg, S.M.; Sequist, L.V. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov. 2014, 4, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Brown, J.M.; Recht, L.; Strober, S. The Promise of Targeting Macrophages in Cancer Therapy. Clin. Cancer Res. 2017, 23, 3241–3250. [Google Scholar] [CrossRef]
- Chen, X.C.; Wei, X.T.; Guan, J.H.; Shu, H.; Chen, D. EGF stimulates glioblastoma metastasis by induction of matrix metalloproteinase-9 in an EGFR-dependent mechanism. Oncotarget 2017, 8, 65969–65982. [Google Scholar] [CrossRef]
- Sørensen, M.D.; Dahlrot, R.H.; Boldt, H.B.; Hansen, S.; Kristensen, B.W. Tumour-associated microglia/macrophages predict poor prognosis in high-grade gliomas and correlate with an aggressive tumour subtype. Neuropathol. Appl. Neurobiol. 2018, 44, 185–206. [Google Scholar] [CrossRef]
- Ding, P.; Wang, W.; Wang, J.; Yang, Z.; Xue, L. Expression of tumor-associated macrophage in progression of human glioma. Cell Biochem. Biophys. 2014, 70, 1625–1631. [Google Scholar] [CrossRef]




| Tumor Group | Entities | Grade |
|---|---|---|
| Diffuse astrocytic and oligodendroglial tumors | Diffuse Astrocytoma, IDH-mutant | II |
| Anaplastic astrocytoma, IDH-mutant | III | |
| Glioblastoma, IDH wildtype | IV | |
| Glioblastoma, IDH mutant | IV | |
| Diffuse midline glioma, H3 K27M-mutant | IV | |
| Oligodendroglioma, IDH-mutant and 1p/19q-codeleted | II | |
| Anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted | III | |
| Other astrocytic tumors | Pilocytic astrocytoma | I |
| Subependymal giant cell astrocytoma | I | |
| Pleomorphic xanthoastrocytoma | II | |
| Anaplastic pleomorphic xanthoastrocytoma | III | |
| Ependymal tumors | Subependymoma | I |
| Myxopapillary ependymoma | I | |
| Ependymoma | II | |
| Ependymoma, RELA fusion-positive | II or III | |
| Anaplastic ependymoma | III | |
| Other gliomas | Angiocentric glioma | I |
| Chordoid glioma of third ventricle | II | |
| Choroid plexus tumors | Chordoid plexux papilloma | I |
| Atypical choroid plexus papilloma | II | |
| Choroid plexus carcinoma | III | |
| Neuronal and mixed neuronal-glial tumors | Dysembryoplastic neuroepithelial tumor | I |
| Gangliocytoma | I | |
| Ganglioglioma | I | |
| Anaplastic ganglioglioma | III | |
| Dystplastic gangliocytoma of cerebellum (Lhermitte-Duclos) | I | |
| Desmoplastic infantile astrocytoma and ganglioglioma | I | |
| Papillary glioneuronal tumor | I | |
| Rosette-forming glioneuronal tumor | I | |
| Central neurocytoma | II | |
| Extraventricular neurocytoma | II | |
| Cerebellar liponeurocytoma | II | |
| Tumours of the pineal region | Pineocytoma | I |
| Pineal parenghymal tumor of intermediate differentiation | II or III | |
| Pineoblastoma | IV | |
| Papillary tumor of the pineal region | II or III | |
| Embryonal tumours | Medulloblastoma (all subtypes) | IV |
| Embryonal tumor with multilayered rosettes, C19MC-altered | IV | |
| Medulloepithelioma | IV | |
| CNS embryonal tumor, NOS | IV | |
| Atypcal teratoid/rhabdoid tumor | IV | |
| CNS embryonal tumor with rhabdoid features | IV | |
| Tumours of the cranial and paraspinal nerves | Schwannoma | I |
| Neurofibroma | I | |
| Perineurioma | I | |
| Malignant peripheral nerve sheath tumor (MPNST) | II, III or IV | |
| Meningiomas | Meningioma | I |
| Atypical meningioma | II | |
| Anaplastic (Malignant) meningioma | III | |
| Mesenchymal, non-meningothelial tumours | Solitary fibrous tumor/haemangiopericytoma | I, II or III |
| Haemangioblastoma | I | |
| Tumors of the sellar region | Craniopharyngioma | I |
| Granular cell tumor | I | |
| Pituicytoma | I | |
| Spindle cell oncocytoma | I |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadagno, E.; Presta, I.; Maisano, D.; Donato, A.; Pirrone, C.K.; Cardillo, G.; Corrado, S.D.; Mignogna, C.; Mancuso, T.; Donato, G.; et al. Role of Macrophages in Brain Tumor Growth and Progression. Int. J. Mol. Sci. 2018, 19, 1005. https://doi.org/10.3390/ijms19041005
Guadagno E, Presta I, Maisano D, Donato A, Pirrone CK, Cardillo G, Corrado SD, Mignogna C, Mancuso T, Donato G, et al. Role of Macrophages in Brain Tumor Growth and Progression. International Journal of Molecular Sciences. 2018; 19(4):1005. https://doi.org/10.3390/ijms19041005
Chicago/Turabian StyleGuadagno, Elia, Ivan Presta, Domenico Maisano, Annalidia Donato, Caterina Krizia Pirrone, Gabriella Cardillo, Simona Domenica Corrado, Chiara Mignogna, Teresa Mancuso, Giuseppe Donato, and et al. 2018. "Role of Macrophages in Brain Tumor Growth and Progression" International Journal of Molecular Sciences 19, no. 4: 1005. https://doi.org/10.3390/ijms19041005