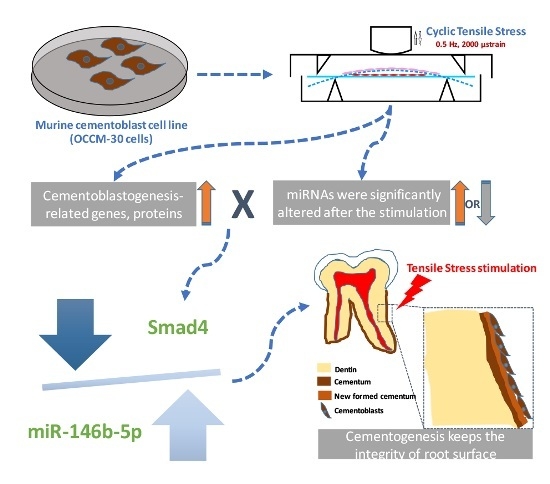
Screening the Expression Changes in MicroRNAs and Their Target Genes in Mature Cementoblasts Stimulated with Cyclic Tensile Stress
Abstract
:1. Introduction
2. Results
2.1. The Effect of Tensile Stress on the Cell Morphology and Proliferation
2.2. Tensile Stress-Induced Osteogenic Differentiation of OCCM-30 Cells
2.3. Identification of Differentially-Expressed miRNAs Caused by Cyclic Tensile Stress
2.4. Validation of the miRNA and mRNA Expression
2.5. Dual-Luciferase Reporter Assay of Direct Binding of miR-146b-5p on the 3′ UTR of Smad4
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Application of Cyclic Tensile Force
4.3. Cell Morphology and Cell Proliferation Assay
4.4. Real-Time PCR Analysis
4.5. Western Blotting
4.6. Alkaline Phosphatase and In Vitro Mineralization Assay
4.7. miRNA Microarray and Bioinformatic Analysis
4.8. Validation of Microarray Results by Quantitative Real-Time PCR
4.9. Dual Luciferase Reporter Assay
4.10. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rego, E.B.; Inubushi, T.; Kawazoe, A.; Miyauchi, M.; Tanaka, E.; Takata, T.; Tanne, K. Effect of pge(2) induced by compressive and tensile stresses on cementoblast differentiation in vitro. Arch. Oral Biol. 2011, 56, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Schroeder, H.E. Cementogenesis reviewed: A comparison between human premolars and rodent molars. Anat. Rec. 1996, 245, 267–292. [Google Scholar] [CrossRef]
- Diercke, K.; Kohl, A.; Lux, C.J.; Erber, R. Il-1β and compressive forces lead to a significant induction of rankl-expression in primary human cementoblasts. J. Orofac. Orthop. 2012, 73, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Diercke, K.; Konig, A.; Kohl, A.; Lux, C.J.; Erber, R. Human primary cementoblasts respond to combined il-1β stimulation and compression with an impaired bsp and cemp-1 expression. Eur. J. Cell Biol. 2012, 91, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Huang, L.; Guo, Y.W.; Cao, L.; Wang, Y.T.; Xu, H.; Bai, D. Expression of alkaline phosphatase in immortalized murine cementoblasts in response to compression-force. Saudi Med. J. 2011, 32, 1235–1240. [Google Scholar] [PubMed]
- Yu, H.; Ren, Y.; Sandham, A.; Ren, A.; Huang, L.; Bai, D. Mechanical tensile stress effects on the expression of bone sialoprotein in bovine cementoblasts. Angle Orthod. 2009, 79, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, E.; Koshikawa, Y.; Kanaya, S.; Tsuchiya, M.; Tamura, M.; Somerman, M.J.; Shimauchi, H. Wnt signaling inhibits cementoblast differentiation and promotes proliferation. Bone 2009, 44, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Miyauchi, M.; Sakamoto, K.; Kitagawa, M.; Noguchi, K.; Somerman, M.J.; Takata, T. Prostaglandin E2 inhibits mineralization and enhances matrix metalloproteinase-13 in mature cementoblasts mainly via the EP4 pathway. Arch. Oral Biol. 2008, 53, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Darendeliler, M.A. Physical properties of root cementum: Part 7. Extent of root resorption under areas of compression and tension. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, N.; Yoshie, H. Occlusion regulates tooth-root elongation during root development in rat molars. Eur. J. Oral. Sci. 2011, 119, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Meng, Y.; Ren, A.; Han, X.; Bai, D.; Bao, L. Response of cementoblast-like cells to mechanical tensile or compressive stress at physiological levels in vitro. Mol. Biol. Rep. 2009, 36, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. Bmp signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Stein, G.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Montecino, M.; Hassan, M.Q.; Gaur, T.; Lengner, C.J.; Young, D.W. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev. Endocr. Metab. Disord. 2006, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012, 23, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, X.; He, Y.; Ye, G.; Li, X.; Zhang, X.; Zhou, L.; Deng, F. Bone morphogenetic protein-9 induces osteogenic differentiation of rat dental follicle stem cells in P38 and ERK1/2 MAPK dependent manner. Int. J. Med. Sci. 2012, 9, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xiao, G.; Berry, J.E.; Franceschi, R.T.; Reddi, A.; Somerman, M.J. Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J. Bone Miner. Res. 2002, 17, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Berry, J.E.; Somerman, M.J. Bone morphogenetic protein-2 inhibits differentiation and mineralization of cementoblasts in vitro. J. Dent. Res. 2003, 82, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Lin, H.; Luo, M.; Wang, J.; Han, G. Integrated mirna and mrna expression profiling of tension force-induced bone formation in periodontal ligament cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.L.; Wang, J.H.; Ding, G.; Yang, S.Y.; Li, Y.; Hu, Y.J.; Wang, S.L. Mechanical force-induced specific microrna expression in human periodontal ligament stem cells. Cells Tissues Organs 2014, 199, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mohammed, A.; Oubaidin, M.; Evans, C.A.; Zhou, X.; Luan, X.; Diekwisch, T.G.; Atsawasuwan, P. Cyclic stretch and compression forces alter microrna-29 expression of human periodontal ligament cells. Gene 2015, 566, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R. Biomechanics and Esthetic Strategies in Clinical Orthodontics; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Shaw, A.M.; Sameshima, G.T.; Vu, H.V. Mechanical stress generated by orthodontic forces on apical root cementum: A finite element model. Orthod. Craniofac. Res. 2004, 7, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Nociti, F.H., Jr.; Foster, B.L.; Tran, A.B.; Dunn, D.; Presland, R.B.; Wang, L.; Bhattacharyya, N.; Collins, M.T.; Somerman, M.J. Vitamin D represses dentin matrix protein 1 in cementoblasts and osteocytes. J. Dent. Res. 2014, 93, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhou, Z.; Huang, L.; Li, Y.; Li, J.; Zou, S. 17β-estradiol regulates the differentiation of cementoblasts via notch signaling cascade. Biochem. Biophys. Res. Commun. 2016, 477, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ahn, Y.H.; Kim, T.H.; Bae, C.H.; Lee, J.C.; You, H.K.; Cho, E.S. Tgf-β signaling regulates cementum formation through osterix expression. Sci. Rep. 2016, 6, 26046. [Google Scholar] [CrossRef] [PubMed]
- Koehne, T.; Jeschke, A.; Petermann, F.; Seitz, S.; Neven, M.; Peters, S.; Luther, J.; Schweizer, M.; Schinke, T.; Kahl-Nieke, B.; et al. Rsk2, the kinase mutated in coffin-lowry syndrome, controls cementum formation. J. Dent. Res. 2016, 95, 752–760. [Google Scholar] [CrossRef] [PubMed]
- D'Errico, J.A.; Berry, J.E.; Ouyang, H.; Strayhorn, C.L.; Windle, J.J.; Somerman, M.J. Employing a transgenic animal model to obtain cementoblasts in vitro. J. Periodontol. 2000, 71, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Qiu, L.; Chang, H.; Yang, Y.; Jian, C.; Xiong, J.; Zhou, J.; Dong, S. Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells. Int. J. Clin. Exp. Pathol. 2014, 7, 7872–7880. [Google Scholar] [PubMed]
- Grottkau, B.E.; Yang, X.; Zhang, L.; Ye, L.; Lin, Y. Comparison of effects of mechanical stretching on osteogenic potential of ascs and bmscs. Bone Res. 2013, 1, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, C.; Han, L.; Liu, L.; Jing, W.; Tang, W.; Tian, W.; Long, J. mir-154-5p regulates osteogenic differentiation of adipose-derived mesenchymal stem cells under tensile stress through the Wnt/PCP pathway by targeting Wnt11. Bone 2015, 78, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Wescott, D.C.; Pinkerton, M.N.; Gaffey, B.J.; Beggs, K.T.; Milne, T.J.; Meikle, M.C. Osteogenic gene expression by human periodontal ligament cells under cyclic tension. J. Dent. Res. 2007, 86, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Kraft, D.C.; Bindslev, D.A.; Melsen, B.; Klein-Nulend, J. Human dental pulp cells exhibit bone cell-like responsiveness to fluid shear stress. Cytotherapy 2011, 13, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Nemoto, E.; Shimauchi, H. Cyclic tensile force up-regulates BMP-2 expression through MAP kinase and COX-2/PGE2 signaling pathways in human periodontal ligament cells. Exp. Cell Res. 2014, 323, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Boabaid, F.; Gibson, C.W.; Kuehl, M.A.; Berry, J.E.; Snead, M.L.; Nociti, F.H., Jr.; Katchburian, E.; Somerman, M.J. Leucine-rich amelogenin peptide: A candidate signaling molecule during cementogenesis. J. Periodontol. 2004, 75, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Shuqin, L.; Shan, Y.; Aishu, R.; Hongwei, D. Investigation of Wnt/β-catenin signaling pathway on regulation of Runx2 in cementoblasts under mechanical stress in vitro. West China J. Stomatol. 2015, 33, 35–39. [Google Scholar]
- Takimoto, A.; Kawatsu, M.; Yoshimoto, Y.; Kawamoto, T.; Seiryu, M.; Takano-Yamamoto, T.; Hiraki, Y.; Shukunami, C. Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development 2015, 142, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, R.; Zhang, H.; Liao, H.; Zhang, Y.; Hinton, R.J.; Feng, J.Q. Osterix controls cementoblast differentiation through downregulation of Wnt-signaling via enhancing DKK1 expression. Int. J. Biol. Sci. 2015, 11, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Torii, D.; Konishi, K.; Watanabe, N.; Goto, S.; Tsutsui, T. Cementogenic potential of multipotential mesenchymal stem cells purified from the human periodontal ligament. Odontology 2015, 103, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hakki, S.S.; Foster, B.L.; Nagatomo, K.J.; Bozkurt, S.B.; Hakki, E.E.; Somerman, M.J.; Nohutcu, R.M. Bone morphogenetic protein-7 enhances cementoblast function in vitro. J. Periodontol. 2010, 81, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wu, B.; Xiao, K.; Kang, J.; Xie, J.; Zhang, X.; Fan, Y. mir-146b-5p promotes metastasis and induces epithelial-mesenchymal transition in thyroid cancer by targeting ZNRF3. Cell. Physiol. Biochem. 2015, 35, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, M.V.; Yamashita, A.S.; Kimura, E.T. Microrna mir-146b-5p regulates signal transduction of TGF-β by repressing SMAD4 in thyroid cancer. Oncogene 2012, 31, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Yu, L.; Sun, C.; Cheng, D.; Yu, S.; Wang, Q.; Yan, Y.; Kang, C.; Jin, S.; et al. mir-146b-5p inhibits glioma migration and invasion by targeting mmp16. Cancer Lett. 2013, 339, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wang, X.; Jie, Z.; Hong, X.; Li, X.; Wang, M.; Yu, Y. Inhibitory effects of mir-146b-5p on cell migration and invasion of pancreatic cancer by targeting MMP16. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Zhao, J.; Ma, J.; Wang, C.; Zhang, C.; Jiang, H.; Cheng, J.; Gao, R.; Zhou, X. mir-146b-5p promotes invasion and metastasis contributing to chemoresistance in osteosarcoma by targeting zinc and ring finger 3. Oncol. Rep. 2016, 35, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-Β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [PubMed]




| Name | Fold Change | p-Value | Up/Down |
|---|---|---|---|
| 6 vs. 0 h | |||
| mmu-miR-146b-5p | 8.149 | 0.0423 | Up |
| mmu-miR-27b-3p | 7.918 | 0.0246 | Up |
| mmu-let-7f-5p | 7.669 | 0.0047 | Up |
| mmu-miR-1893 | 5.628 | 0.0014 | Up |
| mmu-miR-673-3p | 4.688 | 0.0014 | Up |
| mmu-miR-127-5p | 0.057 | 0.0289 | Down |
| mmu-miR-3094-3p | 0.139 | 0.0331 | Down |
| mmu-miR-30c-1-3p | 0.149 | 0.0227 | Down |
| mmu-miR-107-5p | 0.150 | 0.0108 | Down |
| mmu-miR-3106-5p | 0.205 | 0.0048 | Down |
| 18 vs. 0 h | |||
| mmu-miR-3061-3p | 11.470 | 0.0107 | Up |
| mmu-miR-466c-5p | 11.465 | 0.0020 | Up |
| mmu-miR-146b-5p | 11.257 | 0.0469 | Up |
| mmu-miR-1186a | 10.843 | 0.0065 | Up |
| mmu-miR-1893 | 7.214 | 0.0052 | Up |
| mmu-miR-3071-5p | 0.009 | 0.0101 | Down |
| mmu-miR-34c-3p | 0.058 | 0.01162 | Down |
| mmu-miR-1899 | 0.066 | 0.03673 | Down |
| mmu-miR-340-3p | 0.091 | 0.0293 | Down |
| mmu-miR-21a-3p | 0.123 | 0.0373 | Down |
| Signaling Pathway | Example Genes |
|---|---|
| 6 vs. 0 h | |
| MAPK signaling pathway | MAPK1, EGFR, FGF1, FGF10, TGFβ2, TGFβR1 |
| T cell receptor signaling pathway | MAPK1, KRAS, NRAS, IL5, IL2, IL10, SOS1, SOS2 |
| Chemokine signaling pathway | MAPK1, KRAS, NRAS, SOS1, GRB2, PTK2, PIK3R1 |
| Pathways in cancer | SMAD4, RUNX1, TGFβ2, TGFβR1, IGF1R, IGF1, BMP2 |
| Wnt signaling pathway | DKK2, TCF3, APC, DKK3, RUNX2 |
| TGFβ/BMP-Smad signaling pathway | SMAD4, TGFβ2, TGFβR1, BMP2, BMP3 |
| 18 vs. 0 h | |
| MAPK signaling pathway | FGFR1, FGFR2, FGF13, FGF5, TGFβR2, MAP3K1 |
| Pathways in cancer | FGFR1, FGFR2, FGF5, SMAD4, TGFβR2, IGF1, RUNX2 |
| Cytokine-cytokine receptor interaction | PDGFRβ, TGFBβ2, IL1, IL12β, IL6 |
| Wnt signaling pathway | RHOA, APC, RUNX2, SMAD4, FZD4, FZD6, LRP6, DKK2 |
| TGFβ/BMP-Smad signaling pathway | SMAD4, TGFβ2, TGFβR1, BMP2, BMP3 |
| Gene | Primers (5′–3′) | GenBank Acc. No. | Length (bp) |
|---|---|---|---|
| Runx2 | Forward-TTCAACGATCTGAGATTTGTGGG | NM_001145920 | 221 |
| Reverse-GGATGAGGAATGCGCCCTA | |||
| Osterix | Forward-ATGGCGTCCTCTCTGCTTG | NM_130458 | 156 |
| Reverse-TGAAAGGTCAGCGTATGGCTT | |||
| ALP | Forward-GATGTGGAATACGAACTGGATG | NM_007431 | 104 |
| Reverse-TGGGAATGCTTGTGTCTGG | |||
| BSP | Forward-AGAGCGGTGAGTCTAAGGAGT | NM_001033418 | 90 |
| Reverse-TGCCCTTTCCGTTGTTGTCC | |||
| OCN | Forward-ATCTTTCTGCTCACTCTGCTG | NM_001037939 | 117 |
| Reverse-CTTATTGCCCTCCTGCTTGG | |||
| COL-I | Forward-CTGGCGGTTCAGGTCCAAT | NM_007742 | 141 |
| Reverse-TTCCAGGCAATCCACGAGC | |||
| GAPDH | Forward-TGGTGAAGGTCGGTGTGAAC | NM_008084 | 231 |
| Reverse-GCTCCTGGAAGATGGTGATGG | |||
| SMAD4 | Forward-AGGTGGCCTGATCTACACAAG | NM_008540 | 110 |
| Reverse-ACCCGCTCATAGTGATATGGATT |
| Vector Construct | Sequences |
|---|---|
| SMAD4 3′ UTR-WT | F-CCATGCCGAGGAGAGTCAGAGCTGCTGATTGTAAGGCTGAGAAGTTCTCACAGTTAAGCCAC |
| R-TCGAGTGGCTTAACTGTGAGAACTTCTCAGCCTTACAATCAGCAGCTCTGACTCTCCTCGGCATGGAGCT | |
| SMAD4 3′ UTR-MUT | F-CCATGCCGAGGAGAGTCAGAGCTGCTGATTGTAAGGCTGAGATCAAGAGTCAGTTAAGCCAC |
| R-TCGAGTGGCTTAACTGACTCTTGATCTCAGCCTTACAATCAGCAGCTCTGACTCTCCTCGGCATGGAGCT | |
| Mmu-miR-146b inhibitor | F-CAGCCTATGGAATTCAGTTCTCAACCGGTAGCCTATGGAATTCAGTTCTCAC |
| R-TCGAGTGAGAACTGAATTCCATAGGCTACCGGTTGAGAACTGAATTCCATAGGCTGAGCT | |
| Mmu-miR-146b mimics | F-CTGAGAACTGAATTCCATAGGCTACCGGTTGAGAACTGAATTCCATAGGCTC |
| R-TCGAGAGCCTATGGAATTCAGTTCTCAACCGGTAGCCTATGGAATTCAGTTCTCAGAGCT |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Hu, H.; Cheng, Y.; Chen, J.; Bao, C.; Zou, S.; Wu, G. Screening the Expression Changes in MicroRNAs and Their Target Genes in Mature Cementoblasts Stimulated with Cyclic Tensile Stress. Int. J. Mol. Sci. 2016, 17, 2024. https://doi.org/10.3390/ijms17122024
Wang L, Hu H, Cheng Y, Chen J, Bao C, Zou S, Wu G. Screening the Expression Changes in MicroRNAs and Their Target Genes in Mature Cementoblasts Stimulated with Cyclic Tensile Stress. International Journal of Molecular Sciences. 2016; 17(12):2024. https://doi.org/10.3390/ijms17122024
Chicago/Turabian StyleWang, Liao, Haikun Hu, Ye Cheng, Jianwei Chen, Chongyun Bao, Shujuan Zou, and Gang Wu. 2016. "Screening the Expression Changes in MicroRNAs and Their Target Genes in Mature Cementoblasts Stimulated with Cyclic Tensile Stress" International Journal of Molecular Sciences 17, no. 12: 2024. https://doi.org/10.3390/ijms17122024