Mammalian Metallothionein-2A and Oxidative Stress
Abstract
:1. Introduction
2. Metallothionein-2A (MT2A) Gene Expression Regulation
3. MT2A Function
3.1. Anti-Oxidative Stress Injury
3.1.1. The Role of Zn
3.1.2. MT2A and Other Antioxidants
3.2. Anti-Apoptosis
3.3. Anti-Inflammation
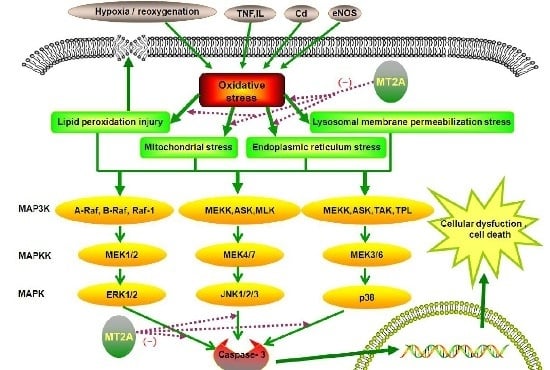
4. MT2A and Oxidative Stress
4.1. Subcellular Changes
4.1.1. Mitochondrial Stress
4.1.2. ER Stress (ERS)
4.1.3. Lysosomal Membrane Permeabilization (LMP) Stress (LMPS)
4.1.4. Biological Membrane Lipid Peroxidation Injury (LPI)
4.2. The Role of MT2A in MAPKs Signals
5. MT2A and Disease
5.1. Cardiovascular Disease
5.2. Nervous System Disease
5.3. Cancers
5.4. Aging
5.5. Diabetes Mellitus
5.6. Hepatal and Gastrointestinal Diseases
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Murakami, S.; Miyazaki, I.; Sogawa, N.; Miyoshi, K.; Asanuma, M. Neuroprotective effects of metallothionein against rotenone-induced myenteric neurodegeneration in parkinsonian mice. Neurotox. Res. 2014, 26, 285–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blindauer, C. Binding, Transport and Storage of Metal Ions in Biological Cells. In Metallothioneins; Maret, W., Wedd, A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2014; pp. 594–653. [Google Scholar]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Su, L.; Yue, H.; Yin, X.; Zhao, J.; Zhang, S.; Kung, H.; Xu, Z.; Miao, J. HMBOX1 interacts with MT2A to regulate autophagy and apoptosis in vascular endothelial cells. Sci. Rep. 2015, 5, 15121. [Google Scholar] [CrossRef] [PubMed]
- Krzeslak, A.; Forma, E.; Chwatko, G.; Jozwiak, P.; Szymczyk, A.; Wilkosz, J.; Rozanski, W.; Brys, M. Effect of metallothionein 2A gene polymorphism on allele-specific gene expression and metal content in prostate cancer. Toxicol. Appl. Pharmacol. 2013, 268, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Krzeslak, A.; Forma, E.; Jozwiak, P.; Szymczyk, A.; Smolarz, B.; Romanowicz-Makowska, H.; Rozanski, W.; Brys, M. Metallothionein 2A genetic polymorphisms and risk of ductal breast cancer. Clin. Exp. Med. 2014, 14, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Vasak, M. Advances in metallothionein structure and functions. J. Trace Elem. Med. Biol. 2005, 19, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chen, L.; Russell, D.H. Metal-induced conformational changes of human metallothionein-2A: A combined theoretical and experimental study of metal-free and partially metalated intermediates. J. Am. Chem. Soc. 2014, 136, 9499–9508. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Positive and negative regulators of the metallothionein gene (review). Mol. Med. Rep. 2015, 12, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.S.; Howells, C.; Eaton, E.D.; Shabala, L.; Zovo, K.; Palumaa, P.; Sillard, R.; Woodhouse, A.; Bennett, W.R.; Ray, S.; et al. The native copper- and zinc-binding protein metallothionein blocks copper-mediated Abeta aggregation and toxicity in rat cortical neurons. PLoS ONE 2010, 5, e12030. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.E.; Bohr, A.; Penkowa, M. The balance between life and death of cells: Roles of metallothioneins. Biomark. Insights 2007, 1, 99–111. [Google Scholar] [PubMed]
- Penkowa, M.; Caceres, M.; Borup, R.; Nielsen, F.C.; Poulsen, C.B.; Quintana, A.; Molinero, A.; Carrasco, J.; Florit, S.; Giralt, M.; et al. Novel roles for metallothionein-I + II (MT-I + II) in defense responses, neurogenesis, and tissue restoration after traumatic brain injury: Insights from global gene expression profiling in wild-type and MT-I + II knockout mice. J. Neurosci. Res. 2006, 84, 1452–1474. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.T.; Hawksworth, G.M.; Beattie, J.H.; Rodilla, V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 35–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Sun, W.; McClung, K.; Pan, Y.; Liang, G.; Tan, Y.; Zhao, Y.; Liu, Q.; Sun, J.; et al. Inhibition of JNK by novel curcumin analog C66 prevents diabetic cardiomyopathy with a preservation of cardiac metallothionein expression. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, R.K.; Chung, R.S.; Penkowa, M. Metallothioneins I and II: Neuroprotective significance during CNS pathology. Int. J. Biochem. Cell Biol. 2007, 39, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Fuquay, R.; Sakurai, T.; Waalkes, M.P. Acquisition of apoptotic resistance in cadmium-induced malignant transformation: Specific perturbation of JNK signal transduction pathway and associated metallothionein overexpression. Mol. Carcinog. 2006, 45, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, S.; Kepinska, M.; Emri, G.; Rodrigo, M.A.; Tmejova, K.; Nerudova, D.; Kizek, R.; Adam, V. Microarray analysis of metallothioneins in human diseases—A review. J. Pharm. Biomed. Anal. 2016, 117, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Cao, Y.; Tian, X.; Zeng, R.; Liao, D.F.; Cao, D. Oxidative Stress and Carbonyl Lesions in Ulcerative Colitis and Associated Colorectal Cancer. Oxid. Med. Cell. Longev. 2016, 2016, 9875298. [Google Scholar] [CrossRef] [PubMed]
- Pagel, J.I.; Deindl, E. Disease progression mediated by egr-1 associated signaling in response to oxidative stress. Int. J. Mol. Sci. 2012, 13, 13104–13117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madureira, P.A.; Waisman, D.M. Annexin A2: The importance of being redox sensitive. Int. J. Mol. Sci. 2013, 14, 3568–3594. [Google Scholar] [CrossRef] [PubMed]
- Cosentino-Gomes, D.; Rocco-Machado, N.; Meyer-Fernandes, J.R. Cell Signaling through Protein Kinase C Oxidation and Activation. Int. J. Mol. Sci. 2012, 13, 10697–10721. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhu, J.; Zhao, X.; Yang, K.; Lu, L.; Zhang, F.; Shen, W.; Zhang, R. All-Trans Retinoic Acid Ameliorates Myocardial Ischemia/Reperfusion Injury by Reducing Cardiomyocyte Apoptosis. PLoS ONE 2015, 10, e0133414. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Mei, W.J.; Xie, Y.Y.; Huang, L.; Yuan, Q.J.; Hu, G.Y.; Tao, L.J.; Peng, Z.Z. Fluorofenidone attenuates oxidative stress and renal fibrosis in obstructive nephropathy via blocking NOX2 (gp91phox) expression and inhibiting ERK/MAPK signaling pathway. Kidney Blood Press. Res. 2015, 40, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, M.; Yazdanparast, R. SK-N-MC cell death occurs by distinct molecular mechanisms in response to hydrogen peroxide and superoxide anions: Involvements of JAK2-STAT3, JNK, and p38 MAP kinases pathways. Cell Biochem. Biophys. 2013, 66, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, J.; Zhao, T.J.; Li, X.H.; Meng, F.G.; Mu, H.; Yan, Y.B.; Zhou, H.M. Metallothioneins protect cytosolic creatine kinases against stress induced by nitrogen-based oxidants. Biochem. J. 2012, 441, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Sun, J.H.; Ke, H.Y.; Chen, Y.J.; Xu, M.; Luo, G.H. Metallothionein 2A genetic polymorphism and its correlation to coronary heart disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3747–3753. [Google Scholar] [PubMed]
- Murphy, E.; Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008, 88, 581–609. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Li, Q.; Sreejayan, N.; Nunn, J.M.; Ren, J. Metallothionein prevents high-fat diet induced cardiac contractile dysfunction: Role of peroxisome proliferator activated receptor gamma coactivator 1alpha and mitochondrial biogenesis. Diabetes 2007, 56, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Koppula, S.; Kim, I.S.; More, S.V.; Kim, B.W.; Choi, D.K. Nuclear factor erythroid 2-related factor 2 signaling in Parkinson disease: A promising multi therapeutic target against oxidative stress, neuroinflammation and cell death. CNS Neurol. Disord. Drug Targets 2012, 11, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Perraut, C.; Pollard, N.; Freedman, J.H. Metallothionein expression and neurocognitive function in mice. Physiol. Behav. 2006, 87, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Starska, K.; Brys, M.; Forma, E.; Olszewski, J.; Pietkiewicz, P.; Lewy-Trenda, I.; Stasikowska-Kanicka, O.; Danilewicz, M.; Krzeslak, A. Metallothionein 2A core promoter region genetic polymorphism and its impact on the risk, tumor behavior, and recurrences of sinonasal inverted papilloma (Schneiderian papilloma). Tumour Biol. 2015, 36, 8559–8571. [Google Scholar] [CrossRef] [PubMed]
- Seibold, P.; Hein, R.; Schmezer, P.; Hall, P.; Liu, J.; Dahmen, N.; Flesch-Janys, D.; Popanda, O.; Chang-Claude, J. Polymorphisms in oxidative stress-related genes and postmenopausal breast cancer risk. Int. J. Cancer 2011, 129, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Tokar, E.J.; Diwan, B.A.; Waalkes, M.P. Early life inorganic lead exposure induces testicular teratoma and renal and urinary bladder preneoplasia in adult metallothionein-knockout mice but not in wild type mice. Toxicology 2010, 276, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Leierer, J.; Rudnicki, M.; Braniff, S.J.; Perco, P.; Koppelstaetter, C.; Muhlberger, I.; Eder, S.; Kerschbaum, J.; Schwarzer, C.; Schroll, A.; et al. Metallothioneins and renal ageing. Nephrol. Dial. Transplant. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R. Metallothionein and the biology of aging. Ageing Res. Rev. 2011, 10, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T.; Demple, B.; Bennett, R.A.; Christiani, D.C.; Fan, R.; Hu, H. Individual variability in the zinc inducibility of metallothionein-IIA mRNA in human lymphocytes. J. Toxicol. Environ. Health A 2000, 61, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Liu, Q.; Cai, L.; Wang, Z.; Feng, W. Stable overexpression of human metallothionein-IIA in a heart-derived cell line confers oxidative protection. Toxicol. Lett. 2009, 188, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Duan, Q.; Lin, L.; Ahmed, M.; Wang, T.; Yao, X. Metallothionein-I/II Knockout Mice Aggravate Mitochondrial Superoxide Production and Peroxiredoxin 3 Expression in Thyroid after Excessive Iodide Exposure. Oxid. Med. Cell. Longev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Artells, E.; Palacios, O.; Capdevila, M.; Atrian, S. Mammalian MT1 and MT2 metallothioneins differ in their metal binding abilities. Metallomics 2013, 5, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Kayaaltı, Z.; Söylemezoğlu, T. The polymorphism of core promoter region on metallothionein 2A-metal binding protein in Turkish population. Mol. Biol. Rep. 2009, 37, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Kukic, I.; Lee, J.K.; Coblentz, J.; Kelleher, S.L.; Kiselyov, K. Zinc-dependent lysosomal enlargement in TRPML1-deficient cells involves MTF-1 transcription factor and ZnT4 (Slc30a4) transporter. Biochem. J. 2013, 451, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Summers, K.L.; Sutherland, D.E.; Stillman, M.J. Single-domain metallothioneins: Evidence of the onset of clustered metal binding domains in Zn-rhMT 1a. Biochemistry 2013, 52, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Giacconi, R.; Muti, E.; Cipriano, C.; Costarelli, L.; Tesei, S.; Gasparini, N.; Malavolta, M. Zinc-bound metallothioneins and immune plasticity: Lessons from very old mice and humans. Immun. Ageing 2007, 4. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, Y.; Xu, W.; Luo, Y.; Hao, J.; Shen, X.L.; Yang, X.; Li, X.; Huang, K. Zinc protects HepG2 cells against the oxidative damage and DNA damage induced by ochratoxin A. Toxicol. Appl. Pharmacol. 2013, 268, 123–131. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Aizenman, E. ERK signaling leads to mitochondrial dysfunction in extracellular zinc-induced neurotoxicity. J. Neurochem. 2010, 114, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Roel, M.; Rubiolo, J.A.; Ternon, E.; Thomas, O.P.; Vieytes, M.R.; Botana, L.M. Crambescin C1 Exerts a Cytoprotective Effect on HepG2 Cells through Metallothionein Induction. Mar. Drugs 2015, 13, 4633–4653. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Zhao, J.; Xu, P.; Wang, Y.; Zhao, J.; Jia, L.; Fu, Z.; Jing, L.; Liu, G.; Peng, S. Protective effects of metallothionein on isoniazid and rifampicin-induced hepatotoxicity in mice. PLoS ONE 2013, 8, e72058. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chitambar, C.R. Role of oxidative stress in the induction of metallothionein-2A and heme oxygenase-1 gene expression by the antineoplastic agent gallium nitrate in human lymphoma cells. Free Radic. Biol. Med. 2008, 45, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Pi, J.; Waalkes, M.P. Metallothionein blocks oxidative DNA damage in vitro. Arch. Toxicol. 2013, 87, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Kim, H.G.; Han, E.H.; Jeong, H.G. Metallothionein-III protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a PI3K and ERK/Nrf2-dependent manner. Toxicol. Appl. Pharmacol. 2008, 231, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.A.; Hidalgo, J.; Manso, Y.; Devisscher, L.; Laukens, D.; Lawrence, D.A. Metallothionein and stress combine to affect multiple organ systems. Cell Stress Chaperones 2014, 19, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, J.; Yang, J.; Schamber, R.; Hu, N.; Nair, S.; Xiong, L.; Ren, J. Antioxidant metallothionein alleviates endoplasmic reticulum stress-induced myocardial apoptosis and contractile dysfunction. Free Radic. Res. 2015, 49, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, R.; Achanzar, W.E.; Qu, W.; Nagamine, T.; Takagi, H.; Mori, M.; Waalkes, M.P. Metallothionein is a potential negative regulator of apoptosis. Toxicol. Sci. 2003, 73, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Inoue, K.; Yanagisawa, R.; Sato, M.; Shimada, A.; Morita, T.; Sawada, M.; Nakamura, K.; Sanbongi, C.; Yoshikawa, T. Protective role of metallothionein in acute lung injury induced by bacterial endotoxin. Thorax 2004, 59, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Lei, X.; Meng, H.; Ouyang, X.; Liang, Z. EOLA1 Inhibits Lipopolysaccharide-Induced Vascular Cell Adhesion Molecule-1 Expression by Association with MT2A in ECV304 Cells. Int. J. Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, N.; Harokopos, V.; Zalevsky, J.; Valavanis, C.; Kotanidou, A.; Szymkowski, D.E.; Kollias, G.; Aidinis, V. Soluble TNF mediates the transition from pulmonary inflammation to fibrosis. PLoS ONE 2006, 1, e108. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, S.; Kong, L.; Chen, J.; Feng, W.; Cai, J.; Miao, L.; Tan, Y. Metallothionein deletion exacerbates intermittent hypoxia-induced renal injury in mice. Toxicol. Lett. 2015, 232, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Fetterman, J.L.; Holbrook, M.; Westbrook, D.G.; Brown, J.A.; Feeley, K.P.; Breton-Romero, R.; Linder, E.A.; Berk, B.D.; Weisbrod, R.M.; Widlansky, M.E.; et al. Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovasc. Diabetol. 2016, 15, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Wang, T.; Zhang, N.; Perera, V.; Liang, X.; Abeysekera, I.R.; Yao, X. Propylthiouracil, Perchlorate, and Thyroid-Stimulating Hormone Modulate High Concentrations of Iodide Instigated Mitochondrial Superoxide Production in the Thyroids of Metallothionein I/II Knockout Mice. Endocrinol. Metab. 2016, 31, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Bragina, O.; Gurjanova, K.; Krishtal, J.; Kulp, M.; Karro, N.; Tougu, V.; Palumaa, P. Metallothionein 2A affects the cell respiration by suppressing the expression of mitochondrial protein cytochrome c oxidase subunit II. J. Bioenerg. Biomembr. 2015, 47, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Li, Y.; Sun, X.; Sun, X. Antiapoptotic effect and inhibition of ischemia/reperfusion-induced myocardial injury in metallothionein-overexpressing transgenic mice. Am. J. Pathol. 2003, 163, 1579–1586. [Google Scholar] [CrossRef]
- Bivik, C.; Ollinger, K. JNK mediates UVB-induced apoptosis upstream lysosomal membrane permeabilization and Bcl-2 family proteins. Apoptosis 2008, 13, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Haberzettl, P.; Hill, B.G. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 2013, 1, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, G.; Wang, Y.; Liu, Q.; Xu, W.; Tan, Y.; Cai, L. Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: Metallothionein protection. J. Cell. Mol. Med. 2009, 13, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Zhang, Q.; Sun, W.; Xin, Y.; Zhang, Z.; Tan, Y.; Zhou, S.; Zhang, C.; Cai, L.; Lu, X.; et al. Zinc treatment prevents type 1 diabetes-induced hepatic oxidative damage, endoplasmic reticulum stress, and cell death, and even prevents possible steatohepatitis in the OVE26 mouse model: Important role of metallothionein. Toxicol. Lett. 2015, 233, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hu, N.; Jiang, S.; Zou, Y.; Yang, J.; Xiong, L.; Ren, J. Heavy metal scavenger metallothionein attenuates ER stress-induced myocardial contractile anomalies: Role of autophagy. Toxicol. Lett. 2014, 225, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Gardner, O.S.; Shiau, C.W.; Chen, C.S.; Graves, L.M. Peroxisome proliferator-activated receptor γ-independent activation of p38 MAPK by thiazolidinediones involves calcium/calmodulin-dependent protein kinase II and protein kinase R: Correlation with endoplasmic reticulum stress. J. Biol. Chem. 2005, 280, 10109–10118. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eno, C.O.; Zhao, G.; Venkatanarayan, A.; Wang, B.; Flores, E.R.; Li, C. Noxa couples lysosomal membrane permeabilization and apoptosis during oxidative stress. Free Radic. Biol. Med. 2013, 65, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Baird, S.K.; Kurz, T.; Brunk, U.T. Metallothionein protects against oxidative stress-induced lysosomal destabilization. Biochem. J. 2006, 394, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ullio, C.; Brunk, U.T.; Urani, C.; Melchioretto, P.; Bonelli, G.; Baccino, F.M.; Autelli, R. Autophagy of metallothioneins prevents TNF-induced oxidative stress and toxicity in hepatoma cells. Autophagy 2015, 11, 2184–2198. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ruiz, A.; Vacio-Adame, P.; Monroy-Noyola, A.; Mendez-Armenta, M.; Ortiz-Plata, A.; Montes, S.; Rios, C. Metallothionein-II inhibits lipid peroxidation and improves functional recovery after transient brain ischemia and reperfusion in rats. Oxid. Med. Cell. Longev. 2014, 20. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Elion, E.A. MAP kinase pathways. J. Cell Sci. 2005, 118, 3569–3572. [Google Scholar] [CrossRef] [PubMed]
- Ceylan-Isik, A.F.; Zhao, P.; Zhang, B.; Xiao, X.; Su, G.; Ren, J. Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis. J. Mol. Cell. Cardiol. 2010, 48, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Tang, Z.; Wang, Y.; Su, G.; Sun, W.; Wei, W.; Li, W.; Miao, L.; Cai, L.; Tan, Y.; et al. Metallothionein prevention of arsenic trioxide-induced cardiac cell death is associated with its inhibition of mitogen-activated protein kinases activation in vitro and in vivo. Toxicol. Lett. 2013, 220, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Peng, L.; Fan, Y.; Zandi, E.; Shertzer, H.G.; Xia, Y. A critical role for IκB kinase β in metallothionein-1 expression and protection against arsenic toxicity. J. Biol. Chem. 2007, 282, 21487–21496. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, J.Y.; Ma, J.; Xu, X.; Wang, Q.; Xiong, L.; Yang, J.; Ren, J. Cardiac-specific overexpression of metallothionein attenuates myocardial remodeling and contractile dysfunction in l-NAME-induced experimental hypertension: Role of autophagy regulation. Toxicol. Lett. 2015, 237, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Nath, R.; Kumar, D.; Li, T.; Singal, P.K. Metallothioneins, oxidative stress and the cardiovascular system. Toxicology 2000, 155, 17–26. [Google Scholar] [CrossRef]
- Giacconi, R.; Muti, E.; Malavolta, M.; Cipriano, C.; Costarelli, L.; Bernardini, G.; Gasparini, N.; Mariani, E.; Saba, V.; Boccoli, G.; et al. The +838 C/G MT2A polymorphism, metals, and the inflammatory/immune response in carotid artery stenosis in elderly people. Mol. Med. 2007, 13, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Doser, T.A.; Fang, C.X.; Nunn, J.M.; Janardhanan, R.; Zhu, M.; Sreejayan, N.; Quinn, M.T.; Ren, J. Metallothionein prolongs survival and antagonizes senescence-associated cardiomyocyte diastolic dysfunction: Role of oxidative stress. FASEB J. 2006, 20, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Ren, J. Endoplasmic reticulum stress impairs murine cardiomyocyte contractile function via an Akt-dependent mechanism. Circulation 2007, 116, 74. [Google Scholar]
- Guo, R.; Ma, H.; Gao, F.; Zhong, L.; Ren, J. Metallothionein alleviates oxidative stress-induced endoplasmic reticulum stress and myocardial dysfunction. J. Mol. Cell. Cardiol. 2009, 47, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, G.; Prass, K.; Priller, J.; Kapinya, K.; Polley, A.; Muselmann, C.; Ruscher, K.; Kannbley, U.; Schmitt, A.O.; Castell, S.; et al. Serial analysis of gene expression identifies metallothionein-II as major neuroprotective gene in mouse focal cerebral ischemia. J. Neurosci. 2002, 22, 5879–5888. [Google Scholar] [PubMed]
- Jakovac, H.; Tota, M.; Grebic, D.; Grubic-Kezele, T.; Barac-Latas, V.; Mrakovcic-Sutic, I.; Milin, C.; Radosevic-Stasic, B. Metallothionein I+II expression as an early sign of chronic relapsing experimental autoimmune encephalomyelitis in rats. Curr. Aging Sci. 2013, 6, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Asanuma, M.; Murakami, S.; Takeshima, M.; Torigoe, N.; Kitamura, Y.; Miyoshi, K. Targeting 5-HT(1A) receptors in astrocytes to protect dopaminergic neurons in Parkinsonian models. Neurobiol. Dis. 2013, 59, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.J.; Quaife, C.J.; Froelick, G.J.; Palmiter, R.D. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J. Nutr. 1996, 126, 1782–1790. [Google Scholar] [PubMed]
- Jin, R.; Chow, V.T.; Tan, P.H.; Dheen, S.T.; Duan, W.; Bay, B.H. Metallothionein 2A expression is associated with cell proliferation in breast cancer. Carcinogenesis 2002, 23, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Jocelyn, K.M.; Yip, G.W.; Bay, B.H. Silencing the Metallothionein-2A gene inhibits cell cycle progression from G1- to S-phase involving ATM and cdc25A signaling in breast cancer cells. Cancer Lett. 2009, 276, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.P.; Hong, Y.H.; Tung, K.Y.; Yang, P.M. In silico and experimental analyses predict the therapeutic value of an EZH2 inhibitor GSK343 against hepatocellular carcinoma through the induction of metallothionein genes. Oncoscience 2016, 3, 9–20. [Google Scholar] [PubMed]
- Mehrian-Shai, R.; Yalon, M.; Simon, A.J.; Eyal, E.; Pismenyuk, T.; Moshe, I.; Constantini, S.; Toren, A. High metallothionein predicts poor survival in glioblastoma multiforme. BMC Med. Genom. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lin, S.; Xing, R.; Zhu, M.; Lin, B.; Cui, J.; Li, W.; Gao, J.; Shen, L.; Zhao, Y.; et al. Epigenetic Upregulation of Metallothionein 2A by Diallyl Trisulfide Enhances Chemosensitivity of Human Gastric Cancer Cells to Docetaxel Through Attenuating NF-κB Activation. Antioxid. Redox Signal. 2016, 24, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Huang, J.; Xing, R.; Yin, X.; Cui, J.; Li, W.; Yu, J.; Lu, Y. Metallothionein 2A inhibits NF-κB pathway activation and predicts clinical outcome segregated with TNM stage in gastric cancer patients following radical resection. J. Transl. Med. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Starska, K.; Krześlak, A.; Forma, E.; Olszewski, J.; Lewy-Trenda, I.; Osuch-Wójcikiewicz, E.; Bryś, M. Genetic polymorphism of metallothionein 2A and risk of laryngeal cancer in a Polish population. Med. Oncol. 2014, 31. [Google Scholar] [CrossRef] [PubMed]
- Habel, N.; Hamidouche, Z.; Girault, I.; Patino-Garcia, A.; Lecanda, F.; Marie, P.J.; Fromigue, O. Zinc chelation: A metallothionein 2A’s mechanism of action involved in osteosarcoma cell death and chemotherapy resistance. Cell Death Dis. 2013, 4, e874. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, K.; Havaki, S.; Kotsinas, A. E2F transcription factors and digestive system malignancies: How much do we know? World J. Gastroenterol. 2014, 20, 10212–10216. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Cipriano, C.; Muti, E.; Malavolta, M. Zinc-binding proteins (metallothionein and alpha-2 macroglobulin) and immunosenescence. Exp. Gerontol. 2006, 41, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Kayaalti, Z.; Sahiner, L.; Durakoglugil, M.E.; Soylemezoglu, T. Distributions of interleukin-6 (IL-6) promoter and metallothionein 2A (MT2A) core promoter region gene polymorphisms and their associations with aging in Turkish population. Arch. Gerontol. Geriatr. 2011, 53, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.J.; de Oliveira, A.R.; Marreiro Ddo, N. Antioxidant role of zinc in diabetes mellitus. World J. Diabetes 2015, 6, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Cipriano, C.; Muti, E.; Costarelli, L.; Maurizio, C.; Saba, V.; Gasparini, N.; Malavolta, M.; Mocchegiani, E. Novel -209A/G MT2A polymorphism in old patients with type 2 diabetes and atherosclerosis: Relationship with inflammation (IL-6) and zinc. Biogerontology 2005, 6, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, K.; Tomioka, S.; Nakajima, K.; Saito, H.; Kato, M.; Kodaira, T.; Yatsuzuka, S.; Shimomura, Y.; Hiroki, T.; Motoyama, K.; et al. Determination of the serum metallothionein (MT)1/2 concentration in patients with Wilson's disease and Menkes disease. J. Trace Elem. Med. Biol. 2014, 28, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, F.; Huang, W.; Kang, Y.J. Metallothionein gene transfection reverses the phenotype of activated human hepatic stellate cells. J. Pharmacol. Exp. Ther. 2013, 346, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Naito, Y.; Takagi, T.; Kugai, M.; Yoriki, H.; Horie, R.; Fukui, A.; Mizushima, K.; Hirai, Y.; Katada, K.; et al. Role of metallothionein in murine experimental colitis. Int. J. Mol. Med. 2013, 31, 1037–1046. [Google Scholar] [PubMed]

 : caused definitely, brown dotted arrows
: caused definitely, brown dotted arrows  : caused indefinitely, Inhibit(−)).
: caused indefinitely, Inhibit(−)).
 : caused definitely, brown dotted arrows
: caused definitely, brown dotted arrows  : caused indefinitely, Inhibit(−)).
: caused indefinitely, Inhibit(−)).
 : caused definitely, brown dotted arrows
: caused definitely, brown dotted arrows  : caused indefinitely, Inhibit(−).)
: caused indefinitely, Inhibit(−).)
 : caused definitely, brown dotted arrows
: caused definitely, brown dotted arrows  : caused indefinitely, Inhibit(−).)
: caused indefinitely, Inhibit(−).)
| Reference | Tissue Type/Sample Size | Findings |
|---|---|---|
| Chung, et al. [10] | Rat: cortical neuron cells;treated with/without Aβ1–40; n = not disclosed | MT-2A was capable of therapeutic approach to AD |
| Yang, et al. [26] | Human: Peripheral blood; 287CHD; 226 control | The gene polymorphism of MT2A-838G/C was correlated to CHD |
| Xu, et al. [65] | Mice: myocytes; 6 wild-type; 6 cardiac-specific MT transgenic mice; Rats: H9c2 and H9c2MT7 cells; n = not disclosed | MT2A could prevent diabetes-induced cardiac ERS, which contributed to prevent DCM |
| Xue, et al. [37] | Rats: H9c2 and H9c2MT7 cells; n = not disclosed | MT2A markedly increased oxidative protection induced by H/R or Cd toxicity in rat cardiac myocytes |
| Jakovac, et al. [87] | Rats: Tissues: spinal cord, liver; BBH and DA | MT2A had neuroprotective role of autoimmune encephalomyelitis |
| Miyazaki, et al. [88] | Mice: Tissues: astrocytes, the striatum; 6-hydroxydopamine-Lesioned parkinsonian model mice; control; n = not disclosed | MT2A provided a promising therapeutic strategy in Parkinson’s disease |
| Pan, et al. [95] | Human: Gastric tumor tissue; 684 GCs patients cohort; 258 GC patients subset | MT2A might be a chemosensitivity indicator in GC patients |
| Kayaalti, et al. [100] | Human: Peripheral blood; 354 individuals aged between 18 and 95 | The IL-6-174C+ carriers and MT2A-5 G-carriers might be more advantageous for longevity |
| Giacconi, et al. [102] | Human: Peripheral blood; 91 Type 2 diabetes patients; 188 control | The MT2A polymorphism was associated with Type 2 diabetes and atherosclerosis |
| Nakazato, et al. [103] | Human: Peripheral blood; 18 chronic hepatitis C patients and 19 Wilson’s disease patients; 200 control | A significantly elevated MT2A was found in patients with chronic hepatitis and Wilson’s disease |
| Xu, et al. [104] | Human: LX-2 cell from human hepatic stellate; pEGFP-N1-hMT-IIA and pEGFP-N1 were transfected into LX-2 cells; n = not disclosed | Liver fibrosis might be treated by MT2A |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, X.-B.; Wei, H.-W.; Wang, J.; Kong, Y.-Q.; Wu, Y.-Y.; Guo, J.-L.; Li, T.-F.; Li, J.-K. Mammalian Metallothionein-2A and Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 1483. https://doi.org/10.3390/ijms17091483
Ling X-B, Wei H-W, Wang J, Kong Y-Q, Wu Y-Y, Guo J-L, Li T-F, Li J-K. Mammalian Metallothionein-2A and Oxidative Stress. International Journal of Molecular Sciences. 2016; 17(9):1483. https://doi.org/10.3390/ijms17091483
Chicago/Turabian StyleLing, Xue-Bin, Hong-Wei Wei, Jun Wang, Yue-Qiong Kong, Yu-You Wu, Jun-Li Guo, Tian-Fa Li, and Ji-Ke Li. 2016. "Mammalian Metallothionein-2A and Oxidative Stress" International Journal of Molecular Sciences 17, no. 9: 1483. https://doi.org/10.3390/ijms17091483