Patient Education Improves Pain and Health-Related Quality of Life in Patients with Established Spinal Osteoporosis in Primary Care—A Pilot Study of Short- and Long-Term Effects
Abstract
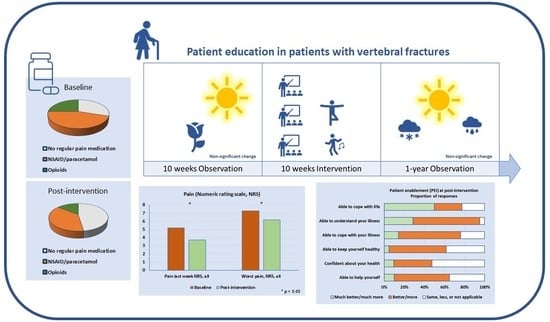
:1. Background
2. Methods
2.1. Study Design
2.2. Participants
2.3. Study Procedures
2.4. Observation
2.5. Interventions
2.6. Outcomes
2.6.1. Pain
2.6.2. Health-Related Quality of Life
2.6.3. Physical Strength, Balance Performance, and Anthropometry
2.6.4. Fall Risk and Physical Activity
2.6.5. Theoretical Knowledge Assessment
2.6.6. Patient Enablement and Overall Experiences of the SOL
3. Statistical Analyses
4. Results
4.1. Background Characteristics
4.2. Observation Period
4.3. Intervention and Long-Term Effect
4.3.1. Pain
4.3.2. Health-Related Quality of Life
4.3.3. Physical Strength, Balance Performance, and Anthropometry
4.3.4. Fall Risk and Physical Activity
4.3.5. Theoretical Knowledge
4.3.6. Patient Enablement and Overall Experiences of the SOL
4.3.7. Adverse Events
5. Discussion
Study Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | activities of daily living |
| EQ-5D | European quality of life |
| FES-I | Swedish Falls Efficacy Scale International |
| HRQoL | health-related quality of life |
| IQR | interquartile range |
| M | mean |
| Md | median |
| PEI | patient enablement instrument |
| SD | standard deviation |
| SOL | school of osteoporosis in Linköping |
| T-group | theory only |
| TMMY-group | theory and mindfulness/medical yoga |
| TPh-group | theory and physical exercise |
| VF | vertebral fracture |
| PF | physical function |
| RF | role physical |
| BP | bodily pain |
| GH | general health |
| VT | vitality |
| SF | social function |
| RE | role emotional |
| MH | mental health |
References
- World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. Report of a WHO Study Group; World Health Organization: Geneva, Switzerland, 1994; Volume 843, pp. 1–129. [Google Scholar]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. Jama 2009, 301, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallberg, I.; Bachrach-Lindström, M.; Hammerby, S.; Toss, G.; Ek, A.-C. Health-related quality of life after vertebral or hip fracture: A seven-year follow-up study. BMC Musculoskelet. Disord. 2009, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden: A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Friesendorff, M.; McGuigan, F.E.; Wizert, A.; Rogmark, C.; Holmberg, A.H.; Woolf, A.D.; Akesson, K. Hip fracture, mortality risk, and cause of death over two decades. Osteoporos. Int. 2016, 27, 2945–2953. [Google Scholar] [CrossRef] [Green Version]
- Johansson, L.; Svensson, H.K.; Karlsson, J.; Olsson, L.E.; Mellström, D.; Lorentzon, M.; Sundh, D. Decreased physical health-related quality of life—A persisting state for older women with clinical vertebral fracture. Osteoporos. Int. 2019, 30, 1961–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söreskog, E.; Ström, O.; Spångéus, A.; Åkesson, K.E.; Borgström, F.; Banefelt, J.; Charokopou, M. Risk of major osteoporotic fracture after first, second and third fracture in Swedish women aged 50 years and older. Bone 2020, 134, 115286. [Google Scholar] [CrossRef]
- Banefelt, J.; Åkesson, K.; Spångéus, A.; Ljunggren, O.; Karlsson, L.; Ström, O.; Ortsäter, G.; Libanati, C.; Toth, E. Risk of imminent fracture following a previous fracture in a Swedish database study. Osteoporos. Int. 2019, 30, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgström, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef]
- Jensen, A.L.; Lomborg, K.; Wind, G.; Langdahl, B.L. Effectiveness and characteristics of multifaceted osteoporosis group education—A systematic review. Osteoporos. Int. 2013, 25, 1209–1224. [Google Scholar] [CrossRef]
- Morfeld, J.C.; Vennedey, V.; Müller, D.; Pieper, D.; Stock, S. Patient education in osteoporosis prevention: A systematic review focusing on methodological quality of randomised controlled trials. Osteoporos. Int. 2017, 28, 1779–1803. [Google Scholar] [CrossRef]
- Rubæk, M.; Hitz, M.F.; Holmberg, T.; Schønwandt, B.M.T.; Andersen, S. Effectiveness of patient education for patients with osteoporosis: A systematic review. Osteoporos. Int. 2021, 33, 959–977. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, J.G.; Drake, M.T.; Sonawane, V.J.; Sinaki, M. Vertebral compression fractures associated with yoga: A case series. Eur. J. Phys. Rehabilitation Med. 2019, 54, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Grahn Kronhed, A.C.; Enthoven, P.; Spångeus, A.; Willerton, C. Mindfulness and modified medical yoga as intervention in older women with osteoporotic vertebral fracture. J. Altern. Complement. Med. 2020, 26, 610–619. [Google Scholar] [CrossRef] [PubMed]
- The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. The rand 36-item health survey 1. 0. Health Econ. 1993, 2, 217–227. [Google Scholar] [CrossRef]
- Orwelius, L.; Nilsson, M.; Nilsson, E.; Wenemark, M.; Walfridsson, U.; Lundström, M.; Kristenson, M. The Swedish RAND-36 Health Survey-reliability and responsiveness assessed in patient populations using Svensson’s method for paired ordinal data. J. Patient-Rep. Outcomes 2018, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Lips, P.; Cooper, C.; Agnusdei, D.; Caulin, F.; Egger, P.; Johnell, O.; Kanis, J.A.; Kellingray, S.; Leplege, A.; Liberman, U.A.; et al. Quality of Life in Patients with Vertebral Fractures: Validation of the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO). Osteoporos. Int. 1999, 10, 150–160. [Google Scholar] [CrossRef]
- Lips, P.; van Schoor, N.M. Quality of life in patients with osteoporosis. Osteoporos. Int. 2005, 16, 447–455. [Google Scholar] [CrossRef]
- Kronhed, A.-C.G.; Möller, C.; Olsson, B.; Möller, M. The Effect of Short-Term Balance Training on Community-Dwelling Older Adults. J. Aging Phys. Act. 2001, 9, 19–31. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s Chair-Stand Test as a Measure of Lower Body Strength in Community-Residing Older Adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Kashman, N.; Volland, G.; Weber, K.; Dowe, M.; Rogers, S. Grip and pinch strength: Normative data for adults. Arch. Phys. Med. Rehabil. 1985, 66, 69–74. [Google Scholar] [PubMed]
- Suwannarat, P.; Amatachaya, P.; Sooknuan, T.; Tochaeng, P.; Kramkrathok, K.; Thaweewannakij, T.; Manimmanakorn, N.; Amatachaya, S. Hyperkyphotic measures using distance from the wall: Validity, reliability, and distance from the wall to indicate the risk for thoracic hyperkyphosis and vertebral fracture. Arch. Osteoporos. 2018, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Nordell, E.; Andreasson, M.; Gall, K.; Thorngren, K.-G. Evaluating the Swedish version of the Falls Efficacy Scale-International (FES-I). Adv. Physiother. 2009, 11, 81–87. [Google Scholar] [CrossRef]
- Olsson, S.J.G.; Ekblom, O.; Andersson, E.; Börjesson, M.; Kallings, L.V. Categorical answer modes provide superior validity to open answers when asking for level of physical activity: A cross-sectional study. Scand. J. Public Health 2015, 44, 70–76. [Google Scholar] [CrossRef]
- Howie, J.G.; Heaney, D.J.; Maxwell, M.; Walker, J.J. A comparison of a Patient Enablement Instrument (PEI) against two established satisfaction scales as an outcome measure of primary care consultations. Fam. Pract. 1998, 15, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enthoven, P.; Peolsson, A.; Ludvigsson, M.L.; Wibault, J.; Peterson, G.; Öberg, B. Validity, internal consistency and self-rated change of the patient enablement instrument in patients with chronic musculoskeletal pain. J. Rehabil. Med. 2019, 51, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rööst, M.; Zielinski, A.; Petersson, C.; Strandberg, E.L. Reliability and applicability of the Patient Enablement Instrument (PEI) in a Swedish general practice setting. BMC Fam. Pract. 2015, 16, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Dawson, B.; Trapp, R. Basic and Clinical Biostatistics, 3rd ed; Lange Medical Books-McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Svensson, H.K.; Olofsson, E.H.; Karlsson, J.; Hansson, T.; Olsson, L.-E. A painful, never ending story: Older women’s experiences of living with an osteoporotic vertebral compression fracture. Osteoporos. Int. 2015, 27, 1729–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronhed, A.-C.G.; Hallberg, I.; Ödkvist, L.; Möller, M. Effect of training on health-related quality of life, pain and falls in osteoporotic women. Adv. Physiother. 2009, 11, 154–165. [Google Scholar] [CrossRef]
- Qvist, N.; Bergström, I.; Kronhed, A.-C.G.; Karlsson, S.; Forss, A. Empowering the fragile body: Experiences of a back muscle group training program in postmenopausal women with vertebral fractures. A qualitative interview study. Adv. Physiother. 2011, 13, 63–70. [Google Scholar] [CrossRef]
- Pietilä Holmner, E.; Stålnacke, B.M.; Enthoven, P.; Stenberg, G. ”The acceptance” of living with chronic pain–an ongoing process: A qualitative study of patient experiences of multimodal rehabilitation in primary care. J. Rehabil. Med. 2018, 50, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherrington, C.; Fairhall, N.; Wallbank, G.; Tiedemann, A.; A Michaleff, Z.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S. Exercise for preventing falls in older people living in the community: An abridged Cochrane systematic review. Br. J. Sports Med. 2019, 54, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, L.M.; Papaioannou, A.; MacIntyre, N.J.; Ashe, M.C.; Heinonen, A.; Shipp, K.; Wark, J.; McGill, S.; Keller, H.; Jain, R.; et al. Too Fit To Fracture: Exercise recommendations for individuals with osteoporosis or osteoporotic vertebral fracture. Osteoporos. Int. 2013, 25, 821–835. [Google Scholar] [CrossRef] [Green Version]
- Varahra, A.; Rodrigues, I.B.; MacDermid, J.C.; Bryant, D.; Birmingham, T. Exercise to improve functional outcomes in persons with osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2018, 29, 265–286. [Google Scholar] [CrossRef]
- Bergland, A.; Thorsen, H.; Kåresen, R. Effect of exercise on mobility, balance, and health-related quality of life in osteoporotic women with a history of vertebral fracture: A randomized, controlled trial. Osteoporos. Int. 2010, 22, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Kessenich, C.R.; Guyatt, G.H.; Patton, C.L.; Griffith, L.E.; Hamlin, A.; Rosen, C.J. Support Group Intervention for Women with Osteoporosis. Rehabil. Nurs. 2000, 25, 88–92. [Google Scholar] [CrossRef]
- The National Board of Health and Welfare. National Guidelines for Musculoskeletal Disorders Rheumatoid Arthritis, Axial Spondyloarthritis, Psoriatic Arthritis, Osteoarthritis and Osteoporosis; Support for Guidance and Management, Report 2021-1-7137; The National Board of Health and Welfare: Stockholm, Sweden, 2021.



| Baseline Md (X) 25–75% | Post-intervention Md (X) 25–75% | p-Value | |
|---|---|---|---|
| Current pain, NRS, All | 3.5 (3.2) 0.3–5.3 | 1.0 (1.9) 0.0–4.3 | 0.093 |
| T group | 2.5 (3.5) 1.0–6.5 | 4.5 (3.0) 0.5–4.8 | 1.000 |
| TPh group | 4.0 (3.7) 1.8–5.0 | 0.0 (1.6) 0.0–3.3 | 0.138 |
| TMMY group | 0.0 (2.4) 0.0–6.0 | 0.0 (1.6) 0.0–3.5 | 0.500 |
| Pain last week, NRS, all | 5.0 (5.2) 4.0–6.9 | 4.5 (3.7) 0.5–5.9 | 0.042 |
| T group | 8.0 (5.9) 2.3–8.5 | 4.5 (5.2) 4.3–6.5 | 0.498 |
| TPh group | 4.5 (4.9) 4.0–6.3 | 2.3 (2.7) 0.0–5.3 | 0.106 |
| TMMY group | 5.0 (5.1) 4.0–6.0 | 5.0 (3.8) 0.0–6.0 | 0.271 |
| Worst pain, NRS, all | 7.8 (7.3) 6.3–8.3 | 6.3 (6.2) 4.9–8.0 | 0.013 |
| T group | 8.0 (8.1) 6.8–9.5 | 7.5 (7.1) 5.8–8.3 | 0.221 |
| TPh group | 6.5 (6.4) 5.0–8.0 | 5.5 (5.0) 3.5–5.5 | 0.058 |
| TMMY group | 8.0 (7.8) 6.9–9.0 | 6.8 (6.8) 5.8–8.0 | 0.223 |
| Baseline (1) Md (X) 25–75% | Post-intervention (2) Md (X) 25–75% | 1-Year Follow-Up (3) Md (X) 25–75% | p-Value (1) vs. (2) | p-Value (2) vs. (3) | ||
|---|---|---|---|---|---|---|
| Health-related quality of life | ||||||
| RAND-36 | ||||||
| Physical function PF, all | 70 (60) 35–85 | 70 (64) 43–85 | 60 (59) 40–85 | 0.060 | 0.146 | |
| T group | 60 (53) 23–80 | 70 (56) 25–80 | 60 (51) 13–85 | 0.180 | 0.257 | |
| TPh group | 70 (62) 40–83 | 80 (70) 53–88 | 65 (66) 43–85 | 0.041 | 0.344 | |
| TMMY group | 70 (62) 40–85 | 70 (62) 40–80 | 60 (56) 35–85 | 0.713 | 0.344 | |
| Role physical RP, all | 25 (39) 0–75 | 38 (49) 0–100 | 38 (49) 0–100 | 0.309 | 1.000 | |
| T group | 25 (25) 0–50 | 25 (40) 0–88 | 0 (40) 0–100 | 0.317 | 1.000 | |
| TPh group | 50 (42) 0–75 | 100 (64) 13–100 | 50 (58) 25–100 | 0.143 | 0.414 | |
| TMMY group | 50 (50) 0–100 | 0 (25) 0–75 | 25 (38) 0–88 | 0.317 | 0.317 | |
| Bodily pain BP, all | 45 (49) 45–63 | 58 (55) 39–78 | 45 (52) 33–68 | 0.163 | 0.443 | |
| T group | 58 (42) 12–64 | 58 (39) 12–58 | 33 (38) 23–57 | 0.785 | 0.786 | |
| TPh group | 45 (48) 45–47 | 68 (64) 45–80 | 58 (60) 45–79 | 0.063 | 0.610 | |
| TMMY group | 55 (56) 35–78 | 45 (55) 33–78 | 55 (50) 25–68 | 0.786 | 0.916 | |
| General health GH, all | 55 (52) 28–73 | 65 (57) 35–73 | 55 (54) 35–70 | 0.203 | 0.154 | |
| T group | 50 (46) 15–76 | 65 (53) 28–73 | 55 (52) 30–73 | 0.345 | 0.891 | |
| TPh group | 55 (54) 25–78 | 60 (58) 35–80 | 55 (56) 33–73 | 0.608 | 0.573 | |
| TMMY group | 50 (55) 45–70 | 65 (59) 35–65 | 50 (53) 35–65 | 0.496 | 0.098 | |
| Vitality VT, all | 55 (55) 33–73 | 60 (58) 38–78 | 60 (60) 43–75 | 0.483 | 0.776 | |
| T group | 40 (49) 35–68 | 55 (52) 28–75 | 60 (53) 35–68 | 1.000 | 1.000 | |
| TPh group | 45 (50) 28–70 | 65 (55) 28–80 | 50 (62) 43–85 | 0.232 | 0.248 | |
| TMMY group | 65 (65) 55–80 | 60 (65) 50–75 | 60 (61) 55–70 | 0.932 | 0.416 | |
| Social function SF, all | 75 (75) 50–100 | 100 (83) 63–100 | 88 (80) 63–100 | 0.048 | 0.476 | |
| T group | 88 (78) 50–100 | 100 (78) 44–100 | 75 (73) 44–100 | 1.000 | 0.414 | |
| TPh group | 63 (74) 57–100 | 100 (85) 63–100 | 88 (85) 69–100 | 0.136 | 0.915 | |
| TMMY group | 75 (73) 50–100 | 88 (84) 63–100 | 88 (80) 50–100 | 0.066 | 0.285 | |
| Role emotional RE, all | 100 (65) 33–100 | 100 (76) 50–100 | 100 (67) 17–100 | 0.262 | 0.395 | |
| T group | 33 (40) 0–84 | 100 (60) 0–100 | 0 (33) 0–84 | 0.414 | 0.180 | |
| TPh group | 100 (67) 33–100 | 100 (78) 50–100 | 100 (81) 67–100 | 0.461 | 0.785 | |
| TMMY group | 100 (100) 33–100 | 100 (100) 50–100 | 100 (78) 33–100 | 1.000. | 0.317 | |
| Mental health MH, all | 76 (73) 66–88 | 80 (77) 66–92 | 80 (75) 60–88 | 0.071 | 0.269 | |
| T group | 68 (66) 52–80 | 80 (74) 50–94 | 60 (66) 56–80 | 0.343 | 0.416 | |
| TPh group | 80 (72) 52–86 | 72 (72) 52–88 | 80 (73) 58–88 | 0.765 | 0.596 | |
| TMMY group | 76 (79) 68–88 | 88 (86) 76–92 | 88 (83) 76–88 | 0.040 | 0.389 | |
| Baseline (1) Md (X) 25–75% | Post-Intervention (2) Md (X) 25–75% | 1-Year Follow-Up (3) Md (X) 25–75% | p-Value (1) vs. (2) | p-Value (2) vs. (3) | ||
|---|---|---|---|---|---|---|
| Health-related quality of life | ||||||
| Qualeffo-41 | ||||||
| Pain, all | 55 (51) 35–65 | 45 (46) 30–67 | 40 (46) 28–68 | 0.143 | 0.615 | |
| T group | 60 (55) 33–75 | 65 (56) 38–70 | 50 (57) 38–80 | 0.786 | 0.854 | |
| TPh group | 60 (56) 45–70 | 40 (45) 35–53 | 35 (41) 23–65 | 0.046 | 0.865 | |
| TMMY group | 40 (42) 25–55 | 38 (40) 30–69 | 40 (44) 35–65 | 0.865 | 0.270 | |
| Activities of daily life, all | 13 (19) 6–25 | 19 (18) 6–19 | 13 (17) 6–22 | 0.709 | 0.326 | |
| T group | 19 (25) 9–44 | 13 (20) 6–38 | 19 (25) 9–44 | 0.581 | 0.461 | |
| TPh group | 13 (16) 6–19 | 19 (15) 6–19 | 13 (12) 6–16 | 0.730 | 0.096 | |
| TMMY group | 13 (18) 6–31 | 19 (19) 13–31 | 19 (17) 6–25 | 0.713 | 0.257 | |
| Jobs around the house, all | 20 (27) 10–45 | 25 (29) 5–50 | 15 (25) 5–48 | 0.417 | 0.075 | |
| T group | 20 (30) 10–55 | 30 (37) 5–73 | 35 (31) 3–58 | 0.257 | 0.705 | |
| TPh group | 10 (18) 5–33 | 5 (17) 5–35 | 10 (13) 3–25 | 0.792 | 0.107 | |
| TMMY group | 45 (37) 10–55 | 50 (39) 15–55 | 45 (35) 10–55 | 0.683 | 0.339 | |
| Mobility, all | 22 (25) 9–46 | 19 (25) 11–38 | 22 (25) 11–33 | 0.659 | 0.587 | |
| T group | 22 (31) 19–48 | 28 (35) 19–55 | 28 (35) 13–61 | 0.180 | 0.854 | |
| TPh group | 25 (25) 13–38 | 19 (22) 11–38 | 22 (23) 13–33 | 0.234 | 0.596 | |
| TMMY group | 9 (21) 6–46 | 13 (21) 6–38 | 16 (20) 6–31 | 0.684 | 0.496 | |
| Social function, all | 40 (41) 22–61 | 33 (34) 17–49 | 37 (37) 15–58 | 0.024 | 0.509 | |
| T group | 53 (55) 43–67 | 43 (49) 33–67 | 43 (51) 36–69 | 0.500 | 0.893 | |
| TPh group | 22 (33) 16–61 | 20 (30) 15–49 | 19 (28) 12–44 | 0.484 | 0.441 | |
| TMMY group | 40 (42) 26–66 | 31 (28) 8–43 | 29 (38) 17–66 | 0.043 | 0.108 | |
| General health perception, all | 58 (56) 38–71 | 58 (52) 42–67 | 58 (53) 38–71 | 0.208 | 0.916 | |
| T group | 58 (67) 50–88 | 67 (67) 54–79 | 58 (58) 33–83 | 1.000 | 0.276 | |
| TPh group | 50 (52) 29–75 | 50 (46) 17–67 | 58 (52) 33–67 | 0.205 | 0.480 | |
| TMMY group | 58 (52) 33–67 | 58 (50) 42–58 | 58 (51) 42–67 | 0.465 | 0.893 | |
| Mental function, all | 36 (39) 31–50 | 42 (40) 22–57 | 36 (46) 26–54 | 0.695 | 0.295 | |
| T group | 44 (53) 36–75 | 33 (41) 25–61 | 53 (47) 25–67 | 0.068 | 1.000 | |
| TPh group | 33 (34) 22–50 | 22 (34) 21–49 | 36 (47) 24–50 | 0.766 | 0.342 | |
| TMMY group | 36 (35) 28–47 | 53 (46) 19–67 | 33 (45) 31–56 | 0.352 | 0.866 | |
| Total score, all | 32 (36) 25–48 | 30 (34) 24–45 | 36 (34) 20–44 | 0.259 | 0.578 | |
| T group | 35 (45) 30–64 | 36 (42) 26–62 | 41 (42) 22–63 | 0.500 | 0.893 | |
| TPh group | 28 (32) 25–42 | 30 (29) 20–40 | 25 (30) 20–42 | 0.192 | 0.678 | |
| TMMY group | 36 (34) 21–45 | 37 (34) 24–46 | 38 (34) 17–45 | 0.612 | 0.612 | |
| EQ-5D, all | ||||||
| EQ-5D index | 0.73 (0.63) 0.62–0.80 | 0.80 (0.72) 0.63–0.80 | 0.75 (0.66) 0.66–0.80 | 0.138 | 0.192 | |
| T group | 0.73 (0.67) 0.57–0.75 | 0.80 (0.62) 0.35–0.80 | 0.73 (0.52) 0.14–0.80 | 0.715 | 0.593 | |
| TPh group | 0.80 (0.54) 0.11–0.80 | 0.80 (0.76) 0.71–0.80 | 0.80 (0.74) 0.66–0.80 | 0.068 | 0.180 | |
| TMMY group | 0.69 (0.74) 0.62–0.85 | 0.73 (0.75) 0.62–0.85 | 0.75 (0.65) 0.54–0.80 | 0.655 | 0.465 | |
| Baseline (1) Md (X) 25–75% | Post-intervention (2) Md (X) 25–75% | p-Value (1) vs. (2) | ||
|---|---|---|---|---|
| Clinical tests | ||||
| Distance C7-wall (cm), all | 6.5 (7.9) 5–11 | 6.5 (7.5) 5–10 | 0.225 | |
| T group | 7 (9.8) 6–15 | 7.5 (10) 5.8–15.5 | 0.854 | |
| TPh group | 8 (8.1) 5–11 | 7 (7.7) 5.3–10.3 | 0.429 | |
| TMMY group | 6 (6.2) 4–6.5 | 5.5 (5.6) 4–6.5 | 0.167 | |
| Hand force right, all | 20 (20) 17–24 | 21 (21) 17–25 | 0.667 | |
| T group | 16 (19) 14–26 | 20 (19) 12–25 | 0.785 | |
| TPh group | 20 (21) 18–23 | 21 (21) 17–24 | 0.596 | |
| TMMY group | 23 (21) 18–27 | 23 (21) 18–26 | 0.257 | |
| Hand force left, all | 18 (19) 14–23 | 18 (20) 15–23 | 0.913 | |
| T group | 13 (16) 11–22 | 20 (18) 11–24 | 0.416 | |
| TPh group | 18 (21) 17–24 | 18 (20) 16–22 | 0.524 | |
| TMMY group | 19 (20) 16–23 | 18 (19) 15–24 | 0.726 | |
| Chair-stand test, all | 9 (9) 7–11 | 13 (12) 11–15 | 0.005 | |
| T group | 9 (9) 6–11 | 12 (10) 5–15 | 0.492 | |
| TPh group | 8 (10) 7–13 | 13 (13) 11–17 | 0.075 | |
| TMMY group | 9 (9) 7–10 | 13 (13) 12–15 | 0.018 | |
| Right leg, eyes open (s), all | 30 (23) 15–30 | 14 (18) 7–30 | 0.019 | |
| T group | 30 (26) 17–30 | 11 (15) 7–26 | 0.109 | |
| TPh group | 18 (18) 10–30 | 13 (14) 4–25 | 0.208 | |
| TMMY group | 30 (28) 30–30 | 30 (24) 11–30 | 0.180 | |
| Left leg, eyes open (s), all | 27 (21) 12–30 | 18 (20) 10–30 | 0.396 | |
| T group | 17 (19) 10–29 | 24 (21) 10–30 | 0.285 | |
| TPh group | 18 (18) 8–30 | 15 (16) 7–24 | 0.340 | |
| TMMY group | 30 (27) 30–30 | 30 (25) 18–30 | 0.180 | |
| Right leg, eyes closed (s), all | 3 (4) 2–5 | 3 (4) 2–6 | 0.289 | |
| T group | 2 (3) 2–7 | 3 (4) 2–6 | 0.785 | |
| TPh group | 2 (2) 1–4 | 3 (3) 2–4 | 0.040 | |
| TMMY group | 4 (5) 3–6 | 4 (5) 3–7 | 0.752 | |
| Left leg, eyes closed (s), all | 2 (3) 1–4 | 3 (5) 2–6 | 0.030 | |
| T group | 2 (3) 2–4 | 2 (4) 1–10 | 0.593 | |
| TPh group | 3 (2) 1–3 | 4 (6) 2–9 | 0.026 | |
| TMMY group | 3 (4) 2–6 | 3 (4) 3–6 | 0.598 | |
| Walking forwards (steps), all | 15 (12) 10–15 | 15 (14) 14–15 | 0.107 | |
| T group | 13 (12) 7–15 | 15 (13) 8–15 | 0.655 | |
| TPh group | 14 (11) 4–15 | 15 (13) 13–15 | 0.068 | |
| TMMY group | 15 (15) 15–15 | 15 (15) 15–15 | 1.000 | |
| Walking backwards (steps), all | 15 (11) 5–15 | 15 (13) 10–15 | 0.027 | |
| T group | 9 (9) 2–15 | 10 (10) 4–15 | 0.180 | |
| TPh group | 15 (11) 4–15 | 15 (13) 9–15 | 0.109 | |
| TMMY group | 15 (14) 15–15 | 15 (14) 15–15 | 0.317 | |
| Weight (kg), all | 63.8 (66.7) 55.8–74.4 | 63.1 (67) 56.7–77.0 | 0.118 | |
| T group | 62.4 (68.8) 54.8–86.1 | 63.1 (69.4) 52.9–89 | 0.500 | |
| TPh group | 67.6 (68.6) 57.2–79.9 | 63.7 (68.5) 57.7–81.1 | 0.594 | |
| TMMY group | 59.4 (62.8) 52.7–70.4 | 58.9 (63.3) 53.6–70.6 | 0.176 | |
| Height (cm), all | 160 (161) 154–169 | 159 (161) 155–170 | 0.517 | |
| T group | 155 (158) 150–167 | 155 (157) 149–168 | 0.492 | |
| TPh group | 164 (164) 157–172 | 164 (163) 157–172 | 0.933 | |
| TMMY group | 156 (159) 154–165 | 157 (159) 154–165 | 0.581 | |
| Baseline (1) Md (X) 25–75% | Post-Intervention (2) Md (X) 25–75% | 1-Year Follow-Up (3) Md (X) 25–75% | p-Value (1) vs. (2) | p-Value (2) vs. (3) | ||
|---|---|---|---|---|---|---|
| Fall | ||||||
| FES-I, all | 21 (26) 19–34 | 22 (26) 20–34 | 24 (26) 20–28 | 0.476 | 0.878 | |
| T group | 24 (30) 19–45 | 26 (30) 20–45 | 28 (33) 18–51 | 0.713 | 0.279 | |
| TPh group | 20 (23) 19–29 | 20 (22) 18–25 | 21 (22) 21–25 | 0.527 | 0.735 | |
| TMMY group | 24 (26) 18–35 | 31 (28) 20–35 | 27 (24) 19–29 | 0.236 | 0.207 | |
| Physical activity | ||||||
| Physical exercise weekly (min), all | 30 (42) 8–75 | 45 (41) 0–75 | 45 (51) 0–98 | 0.524 | 0.086 | |
| T group | 30 (39) 30–53 | 45 (39) 0–75 | 45 (57) 23–98 | 0.890 | 0.336 | |
| TPh group | 15 (38) 0–75 | 38 (39) 0–68 | 30 (41) 0–90 | 1.000 | 0.705 | |
| TMMY group | 45 (49) 30–75 | 45 (43) 0–75 | 75 (58) 0–105 | 0.414 | 0.102 | |
| Everyday activity weekly (min), all | 225 (198) 98–300 | 225 (200) 98–300 | 225 (189) 83–263 | 0.863 | 0.552 | |
| T group | 120 (138) 60–225 | 225 (195) 75–300 | 225 (168) 45–263 | 0.066 | 0.102 | |
| TPh group | 300 (205) 45–300 | 225 (195) 75–300 | 225 (178) 38–263 | 0.498 | 0.593 | |
| TMMY group | 225 (231) 225–300 | 225 (210) 120–300 | 225 (216) 120–300 | 0.671 | 1.000 | |
| Total physical activity weekly (min), all | 255 (235) 116–323 | 255 (236) 128–334 | 255 (247) 146–345 | 0.948 | 0.628 | |
| T group | 150 (177) 90–278 | 270 (234) 75–375 | 225 (225) 90–360 | 0.144 | 0.684 | |
| TPh group | 263 (231) 49–375 | 232 (221) 101–326 | 255 (236) 79–371 | 0.610 | 0.750 | |
| TMMY group | 270 (281) 225–330 | 300 (253) 195–300 | 300 (274) 225–345 | 0.611 | 0.465 | |
| Daily sitting/resting (hours), all | 5 (5) 2–7 | 5 (5) 2–7 | 5 (5) 2–5 | 0.796 | 0.655 | |
| T group | 2 (4) 2–7 | 5 (4) 4–5 | 5 (5) 4–7 | 0.564 | 0.317 | |
| TPh group | 5 (5) 2–7 | 5 (5) 2–8 | 5 (4) 2–5 | 0.527 | 0.317 | |
| TMMY group | 5 (6) 5–8 | 5 (6) 5–8 | 5 (6) 5–8 | 1.000 | 1.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spångeus, A.; Willerton, C.; Enthoven, P.; Grahn Kronhed, A.-C. Patient Education Improves Pain and Health-Related Quality of Life in Patients with Established Spinal Osteoporosis in Primary Care—A Pilot Study of Short- and Long-Term Effects. Int. J. Environ. Res. Public Health 2023, 20, 4933. https://doi.org/10.3390/ijerph20064933
Spångeus A, Willerton C, Enthoven P, Grahn Kronhed A-C. Patient Education Improves Pain and Health-Related Quality of Life in Patients with Established Spinal Osteoporosis in Primary Care—A Pilot Study of Short- and Long-Term Effects. International Journal of Environmental Research and Public Health. 2023; 20(6):4933. https://doi.org/10.3390/ijerph20064933
Chicago/Turabian StyleSpångeus, Anna, Catrin Willerton, Paul Enthoven, and Ann-Charlotte Grahn Kronhed. 2023. "Patient Education Improves Pain and Health-Related Quality of Life in Patients with Established Spinal Osteoporosis in Primary Care—A Pilot Study of Short- and Long-Term Effects" International Journal of Environmental Research and Public Health 20, no. 6: 4933. https://doi.org/10.3390/ijerph20064933