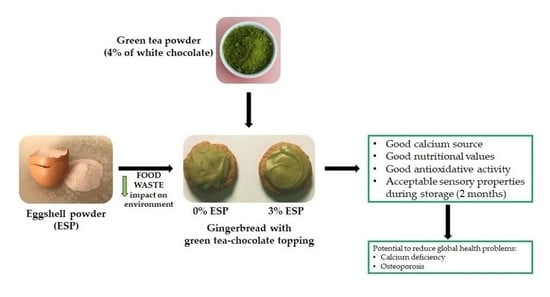
Innovative Application of Chicken Eggshell Calcium to Improve the Functional Value of Gingerbread
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation of Eggshell Powder and Gingerbread
2.2. Basic Composition Analysis
2.3. Total Phenolic Content and Antioxidative Activity Analysis
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Basic Composition Analysis
3.2. Total Phenolic Content and Antioxidative Activity Analysis
3.3. Sensory Analysis
3.3.1. Sensory Analysis of Fresh Gingerbread Samples
3.3.2. Hedonic Evaluation during Storage Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kim, M.H.; Lee, H.J. Osteoporosis, Vitamin C Intake, and Physical Activity in Korean Adults Aged 50 Years and Over. J. Phys. Ther. Sci. 2016, 28, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Wawrzyniak, N.; Suliburska, J.; Kulczyński, B.; Kołodziejski, P.; Kurzawa, P.; Gramza-Michałowska, A. Calcium-Enriched Pumpkin Affects Serum Leptin Levels and Fat Content in a Rat Model of Postmenopausal Osteoporosis. Nutrients 2021, 13, 2334. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Suliburska, J.; Rybarczyk, M.; Gramza-Michałowska, A. The Effect of Osmotic Dehydration Conditions on the Calcium Content in Plant Matrice. Food Chem. 2021, 343, 128519. [Google Scholar] [CrossRef] [PubMed]
- Brun, L.R.; Lupo, M.; Delorenzi, D.A.; Di Loreto, V.E.; Rigalli, A. Chicken Eggshell as Suitable Calcium Source at Home. Int. J. Food Sci. Nutr. 2013, 64, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.M. Chicken Eggshell Powder as Dietary Calcium Source in Biscuits. World J. Dairy Food Sci. 2015, 10, 199–206. [Google Scholar]
- Al Mijan, M.; Choi, K.H.; Kwak, H.S. Physicochemical, Microbial, and Sensory Properties of Nanopowdered Eggshell-Supplemented Yogurt during Storage. J. Dairy Sci. 2014, 97, 3273–3280. [Google Scholar] [CrossRef] [PubMed]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szymandera-Buszka, K.; Rezler, R.; Jarzębski, M.; Szczepaniak, O.; Marciniak, G.; Jędrusek-Golińska, A.; Kobus-Moryson, M. Effect of Fortification with Calcium from Eggshells on Bioavailability, Quality, and Rheological Characteristics of Traditional Polish Bread Spread. J. Dairy Sci. 2020, 103, 6918–6929. [Google Scholar] [CrossRef]
- Schaafsma, A.; Beelen, G.M. Eggshell Powder, a Comparable or Better Source of Calcium than Purified Calcium Carbonate: Piglet Studies. J. Sci. Food Agric. 1999, 79, 1596–1600. [Google Scholar] [CrossRef]
- Siddique, S.; Firdous, S.; Durrani, A.I.; Khan, S.J.; Saeed, A. Hesperidin, a Citrus Flavonoid, Increases the Bioavailability of Micronutrients of Gallus Domesticus (Chicken) Eggshell: In Vitro Study. Chem. Speciat. Bioavailab. 2016, 28, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Murakami, F.S.; Rodrigues, P.O.; De Campos, C.M.T.; Silva, M.A.S. Physicochemical Study of CaCO3 from Egg Shells. Food Sci. Technol. 2007, 27, 658–662. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.A.; Benelli, P.; Amante, E.R. A Literature Review on Adding Value to Solid Residues: Egg Shells. J. Clean. Prod. 2013, 46, 42–47. [Google Scholar] [CrossRef]
- Valenzuela, F.; Böhm, S. Against Wasted Politics: A Critique of the Circular Economy. Ephemer. Theory Polit. Organ. 2017, 17, 23–60. [Google Scholar]
- FAO. Livestock Primary. Available online: http://www.fao.org/faostat/en/?#data/QL (accessed on 10 November 2021).
- Quina, M.J.; Soares, M.A.R.; Quinta-Ferreira, R. Applications of Industrial Eggshell as a Valuable Anthropogenic Resource. Resour. Conserv. Recycl. 2017, 123, 176–186. [Google Scholar] [CrossRef]
- Abdi, E.; Gharachorloo, M.; Ghavami, M. Investigation of Using Egg Shell Powder for Bleaching of Soybean Oil. LWT—Food Sci. Technol. 2021, 140, 110859. [Google Scholar] [CrossRef]
- Aditya, S.; Stephen, J.; Radhakrishnan, M. Utilization of Eggshell Waste in Calcium-Fortified Foods and Other Industrial Applications: A Review. Trends Food Sci. Technol. 2021, 115, 422–432. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative Stress in Bone Remodeling: Role of Antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Arnold, M.; Rajagukguk, Y.V.; Gramza-Michałowska, A. Functional Food for Elderly High in Antioxidant and Chicken Eggshell Calcium to Reduce the Risk of Osteoporosis—A Narrative Review. Foods 2021, 10, 656. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D.; Korczak, J.; Helak, B.; Dziedzic, K.; Górecka, D. Antioxidative Potential, Nutritional Value and Sensory Profiles of Confectionery Fortified with Green and Yellow Tea Leaves (Camellia Sinensis). Food Chem. 2016, 211, 448–454. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kochman, J.; Kwiatkowska, A.; Kałdunska, J.; Dec, K.; Kawczuga, D.; Janda, K. Antioxidant Properties and Nutritional Composition of Matcha Green Tea. Foods 2020, 9, 483. [Google Scholar] [CrossRef]
- Li, X.; Qiao, Y.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Yan, S.; Xie, X.; Huang, D.; et al. Tea Consumption and Bone Health in Chinese Adults: A Population-Based Study. Osteoporos. Int. 2019, 30, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Wang, L.; Ma, Q.; Cui, Q.; Lv, Q.; Zhang, W.; Li, X. Association between Tea Consumption and Osteoporosis: A Meta-Analysis. Medicine 2017, 96, e9034. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tang, R. Tea Drinking Habits and Osteoporotic Hip/Femur Fractures: A Case-Control Study. Pak. J. Med. Sci. 2016, 32, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yang, Y.C.; Yao, W.J.; Lu, F.H.; Wu, J.S.; Chang, C.J. Epidemiological Evidence of Increased Bone Mineral Density in Habitual Tea Drinkers. Arch. Intern. Med. 2002, 162, 1001–1006. [Google Scholar] [CrossRef] [Green Version]
- Muraki, S.; Yamamoto, S.; Ishibashi, H.; Oka, H.; Yoshimura, N.; Kawaguchi, H.; Nakamura, K. Diet and Lifestyle Associated with Increased Bone Mineral Density: Cross-Sectional Study of Japanese Elderly Women at an Osteoporosis Outpatient Clinic. J. Orthop. Sci. 2007, 12, 317–320. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Maghbooli, Z.; Shafaei, A.R.; Javadi, E.; Larijani, B. Relationship between Tea Drinking and Bone Mineral Density in Iranian Population. Iran. J. Public Health 2007, 36 (Suppl S1), 57–62. [Google Scholar]
- Shen, C.L.; Wang, P.; Guerrieri, J.; Yeh, J.K.; Wang, J.S. Protective Effect of Green Tea Polyphenols on Bone Loss in Middle-Aged Female Rats. Osteoporos. Int. 2008, 19, 979–990. [Google Scholar] [CrossRef]
- Lin, S.Y.; Kan, J.Y.; Lu, C.C.; Huang, H.H.; Cheng, T.L.; Huang, H.T.; Ho, C.J.; Lee, T.C.; Chuang, S.C.; Lin, Y.S.; et al. Green Tea Catechin (-)-Epigallocatechin-3-Gallate (EGCG) Facilitates Fracture Healing. Biomolecules 2020, 10, 620. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A. Caffeine in Tea Camellia Sinensis—Content, Absorption, Benefits and Risks of Consumption. J. Nutr. Health Aging 2014, 18, 143–149. [Google Scholar] [CrossRef]
- Bobecka, P. Pierniki Toruńskie w Historii i w Podaniach Lokalnych. Rocz. Toruński 2012, 39, 15–42. [Google Scholar]
- Castella, K. A World of Cake: 150 Recipes for Sweet Traditions from Cultures Near and Far-Honey Cakes to Flat Cakes, Fritters to Chiffons, Meringues to Mooncakes, Tartes to Tortes, Fruit Cakes to Spice Cakes; Sutherland, M., Ringer, N.W., Eds.; Storey Publishing: North Adams, MA, USA, 2010. [Google Scholar]
- Levytska, N.; Kotsiubanska, O. Confectionery in Ukraine: Adoption of European Food Tradition. Probl. Humanit. Hist. Ser. Collect. Sci. Work. Drohobych Ivan Franko State Pedagog. Univ. 2020, 3, 272–282. [Google Scholar] [CrossRef]
- Eurostat. The Gingerbread Tradition is Diminishing. Available online: https://ec.europa.eu/eurostat/fr/web/products-eurostat-news/-/DDN-20201222-1 (accessed on 8 December 2021).
- Masood, S.; Rehman, A.ur.; Bashir, S.; El Shazly, M.; Imran, M.; Khalil, P.; Ifthikar, F.; Jaffar, H.M.; Khursheed, T. Investigation of the Anti-Hyperglycemic and Antioxidant Effects of Wheat Bread Supplemented with Onion Peel Extract and Onion Powder in Diabetic Rats. J. Diabetes Metab. Disord. 2021, 20, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza, S.; Roncero-Ramos, I.; Rufián-Henares, J.Á.; Delgado-Andrade, C. Antioxidant Balance after Long-Term Consumption of Standard Diets Including Bread Crust Glycated Compounds by Adult Rats. Food Res. Int. 2014, 64, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Riboldi, B.P.; Vinhas, Á.M.; Moreira, J.D. Risks of Dietary Acrylamide Exposure: A Systematic Review. Food Chem. 2014, 157, 310–322. [Google Scholar] [CrossRef]
- Amrein, T.M.; Schönbächler, B.; Escher, F.; Amadò, R. Acrylamide in Gingerbread: Critical Factors for Formation and Possible Ways for Reduction. J. Agric. Food Chem. 2004, 52, 4282–4288. [Google Scholar] [CrossRef]
- Kozakai, T.; Uozumi, N.; Katoh, K.; Obara, Y. Dietary Magnesium Increases Calcium Absorption of Ovine Small Intestine in Vivo and in Vitro. Reprod. Nutr. Dev. 2002, 42, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.M. Diet, Gut Microbiome, and Bone Health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef]
- Ray, S.; Barman, A.K.; Roy, P.K.; Singh, B.K. Chicken Eggshell Powder as Dietary Calcium Source in Chocolate Cakes. Pharma Innov. J. 2017, 6, 1–4. [Google Scholar]
- Ahmad, M.; Baba, W.N.; Wani, T.A.; Gani, A.; Gani, A.; Shah, U.; Wani, S.M.; Masoodi, F.A. Effect of Green Tea Powder on Thermal, Rheological & Functional Properties of Wheat Flour and Physical, Nutraceutical & Sensory Analysis of Cookies. J. Food Sci. Technol. 2015, 52, 5799–5807. [Google Scholar]
- PN-EN ISO 3947; Starches, Native Or Modified—Determination Of Total Fat Content; ISO: Geneva, Switzerland, 2001.
- AOAC. Official Methods of Analysis: Official Method for Protein; Method No. 920.87; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- AOAC. Official Methods of Analysis: Official Method for Ash; Method No. 936.03; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011; EUR-Lex: Brussels, Belgium, 2011.
- Dziedzic, K.; Górecka, D.; Kucharska, M.; Przybylska, B. Influence of Technological Process during Buckwheat Groats Production on Dietary Fibre Content and Sorption of Bile Acids. Food Res. Int. 2012, 47, 279–283. [Google Scholar] [CrossRef]
- Suliburska, J.; Krejpcio, Z. Evaluation of the Content and Bioaccessibility of Iron, Zinc, Calcium and Magnesium from Groats, Rice, Leguminous Grains and Nuts. J. Food Sci. Technol. 2014, 51, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Naczk, M. Methods of Analysis and Quantification of Phenolic Compounds. In Food Phenolic: Sources, Chemistry, Effects and Applications; Technomic Publishing Company: Lancaster, PA, USA, 1995; pp. 287–293. [Google Scholar]
- Jan, U.; Gani, A.; Ahmad, M.; Shah, U.; Baba, W.N.; Masoodi, F.A.; Maqsood, S.; Gani, A.; Wani, I.A.; Wani, S.M. Characterization of Cookies Made from Wheat Flour Blended with Buckwheat Flour and Effect on Antioxidant Properties. J. Food Sci. Technol. 2015, 52, 6334–6344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Methods for the Assessment of Antioxidant Activity in Foods. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 287–333. [Google Scholar]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Korczak, J. Oxygen Radical Absorbance Capacity of Selected Food Products. Acta Sci. Pol. Technol. Aliment. 2013, 12, 175–180. [Google Scholar]
- Gramza-Michalowska, A.; Sidor, A.; Regula, J.; Kulczynski, B. PCL Assay Application in Superoxide Anion-Radical Scavenging Capacity of Tea Camellia Sinensis Extracts. Acta Sci. Pol. Technol. Aliment. 2015, 14, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Besco, E.; Braccioli, E.; Vertuani, S.; Ziosi, P.; Brazzo, F.; Bruni, R.; Sacchetti, G.; Manfredini, S. The Use of Photochemiluminescence for the Measurement of the Integral Antioxidant Capacity of Baobab Products. Food Chem. 2007, 102, 1352–1356. [Google Scholar] [CrossRef]
- Rajagukguk, Y.V.; Arnold, M.; Sidor, A.; Kulczyński, B.; Brzozowska, A.; Schmidt, M.; Gramza-Michałowska, A. Antioxidant Activity, Probiotic Survivability, and Sensory Properties of a Phenolic-Rich Pulse Snack Bar Enriched with Lactiplantibacillus plantarum. Foods 2022, 11, 309. [Google Scholar] [CrossRef]
- Gacula, M.C., Jr.; Singh, J.; Bi, J.; Altan, S. Statistical Methods in Food and Consumer Research, 2nd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Garkina, P.K.; Kurochkin, A.A.; Frolov, D.I.; Shaburova, G.V. Effect of Addition Corn Flour, Millet Flour and Pumpkin Seed Flour on the Properties of Gluten-Free Gingerbread. IOP Conf. Ser. Earth Environ. Sci. 2021, 845, 012108. [Google Scholar] [CrossRef]
- Alsuhaibani, A.M.A. Rheological and Nutritional Properties and Sensory Evaluation of Bread Fortified with Natural Sources of Calcium. J. Food Qual. 2018, 2018, 8308361. [Google Scholar] [CrossRef] [Green Version]
- Chilek, T.Z.T.; Kairuaman, N.A.; Ahmad, F.; Wahab, R.A.; Zamri, A.I.; Mahmood, A. Development of White Bread Fortified with Calcium Derived from Eggshell Powder. Malays. Appl. Biol. 2018, 47, 29–39. [Google Scholar]
- Khan, F.A.; Ameer, K.; Qaiser, M.A.; Pasha, I.; Mahmood, Q.; Anjum, F.M.; Riaz, A.; Amir, R.M. Development and Analysis of Bread Fortified with Calcium Extracted from Chicken Eggshells of Pakistani Market. Food Sci. Technol. 2021, 41, 14–20. [Google Scholar] [CrossRef]
- Afzal, F.; Mueen-ud-Din, G.; Nadeem, M.; Murtaza, M.A.; Mahmood, S. Effect of Eggshell Powder Fortification on the Physicochemical and Organoleptic Characteristics of Muffins. Pure Appl. Biol. 2020, 9, 1488–1496. [Google Scholar] [CrossRef]
- FDA. Daily Value and Percent Daily Value: Changes on the New Nutrition and Supplement Facts Labels Daily. Available online: https://www.fda.gov/media/135301/download (accessed on 14 November 2021).
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and Functional Characteristics of Dietary Fibre in Beans, Lentils, Peas and Chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Rajagukguk, Y.V.; Arnold, M.; Gramza-Michałowska, A. Pulse Probiotic Superfood as Iron Status Improvement Agent in Active Women—A Review. Molecules 2021, 26, 2121. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Chiang, W.D.; Thomas, R.L.; Kunkel, M.E. Calcium Binding by Cellulose and Lignin. Food Chem. 1994, 50, 191–196. [Google Scholar] [CrossRef]
- Platt, S.R.; Clydesdale, F.M. Mineral Binding Characteristics of Lignin, Guar Gum, Cellulose, Pectin and Neutral Detergent Fiber under Simulated Duodenal PH Conditions. J. Food Sci. 1987, 52, 1414–1419. [Google Scholar] [CrossRef]
- Al-awwal, N.Y.; Ali, U.L. Proximate Analyses of Different Samples of Egg Shells Obtained from Sokoto Market in Nigeria. Int. J. Sci. Res. 2015, 4, 564–566. [Google Scholar]
- Ajala, E.O.; Eletta, O.A.A.; Ajala, M.A.; Oyeniyi, S.K. Characterization and Evaluation of Chicken Eggshell for Use as a Bio-Resource. Arid. Zo. J. Eng. Technol. Environ. 2018, 14, 26–40. [Google Scholar]
- Nilsuwan, K.; Arnold, M.; Benjakul, S.; Prodpran, T.; de la Caba, K. Properties of Chicken Protein Isolate/Fish Gelatin Blend Film Incorporated with Phenolic Compounds and Its Application as Pouch for Packing Chicken Skin Oil. Food Packag. Shelf Life 2021, 30, 100761. [Google Scholar] [CrossRef]
- Thakur, R.J.; Shaikh, H.; Gat, Y.; Waghmare, R.B. Effect of Calcium Chloride Extracted from Eggshell in Maintaining Quality of Selected Fresh-Cut Fruits. Int. J. Recycl. Org. Waste Agric. 2019, 8, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Repon, K.S.; Sultana, K.; Nahar, K. Identification of Functional Molecules and Pharmacological Activities Investigation of the Methanolic Extract of Egg Shell of Gallus Gallus Domesticus. Mod. Appl. Bioequivalence Bioavailab. 2017, 1, 555561. [Google Scholar]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Antioxidant Activity of Enzymatic Hydrolysates from Eggshell Membrane Proteins and Its Protective Capacity in Human Intestinal Epithelial Caco-2 Cells. J. Funct. Foods 2014, 10, 35–45. [Google Scholar] [CrossRef]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Peptides Derived from Eggshell Membrane Improve Antioxidant Enzyme Activity and Glutathione Synthesis against Oxidative Damage in Caco-2 Cells. J. Funct. Foods 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Olszowy, M.; Dawidowicz, A.L. Is It Possible to Use the DPPH and ABTS Methods for Reliable Estimation of Antioxidant Power of Colored Compounds? Chem. Pap. 2018, 72, 393–400. [Google Scholar] [CrossRef]
- Perez-Perez, L.M.; Huerta-Ocampo, J.Á.; Ruiz-Cruz, S.; Cinco-Moroyoqui, F.J.; Wong-Corral, F.J.; Rascón-Valenzuela, L.A.; Robles-García, M.A.; González-Vega, R.I.; Rosas-Burgos, E.C.; Corella-Madueño, M.A.G.; et al. Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer Arietinum L. Cv Blanoro) Stored under N2 and CO2 Atmospheres. Molecules 2021, 26, 2773. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Saljoughian, M. An Overview of Antioxidants. US Pharm. 2008, 12 (Suppl), 8–11. [Google Scholar]
- Mannucci, A.; Castagna, A.; Santin, M.; Serra, A.; Mele, M.; Ranieri, A. Quality of Flaxseed Oil Cake under Different Storage Conditions. LWT 2019, 104, 84–90. [Google Scholar] [CrossRef]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. γ-Tocopherol, the Major Form of Vitamin E in the US Diet, Deserves More Attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Jin, S.; Song, H.; Huang, K.; Li, S.; Guan, X.; Wang, Y. Effect of Extrusion Pretreatment on Extraction, Quality and Antioxidant Capacity of Oat (Avena Sativa L.) Bran Oil. J. Cereal Sci. 2020, 95, 102972. [Google Scholar] [CrossRef]
- Berker, K.I.; Ozdemir Olgun, F.A.; Ozyurt, D.; Demirata, B.; Apak, R. Modified Folin-Ciocalteu Antioxidant Capacity Assay for Measuring Lipophilic Antioxidants. J. Agric. Food Chem. 2013, 61, 4783–4791. [Google Scholar] [CrossRef]
- Abdullakasim, P.; Songchitsomboon, S.; Techagumpuch, M.; Balee, N.; Swatsitang, P.; Sungpuag, P. Antioxidant Capacity, Total Phenolics and Sugar Content of Selected Thai Health Beverages. Int. J. Food Sci. Nutr. 2007, 58, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, V.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Optimization of Extraction Solvent and Fast Blue BB Assay for Comparative Analysis of Antioxidant Phenolics from Cucumis Melo L. Plants 2021, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Amigo-Benavent, M.; Zielinski, H.; del Castillo, M.D. Effect of Bread Making on Formation of Maillard Reaction Products Contributing to the Overall Antioxidant Activity of Rye Bread. J. Cereal Sci. 2008, 48, 123–132. [Google Scholar] [CrossRef]
- Purlis, E. Browning Development in Bakery Products—A Review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Kumari, S.; Raj, J.D.; Shukla, R.N. Study of Quality & Antioxidant Properties of Green Tea Doughnut. J. Pharmacogn. Phytochem. 2018, 7, 3101–3106. [Google Scholar]
- Xia, Q.; Liu, C.; Liu, J.; Pan, W.; Lu, X.; Yang, J.; Chen, W.; Zheng, L. Rapid and Non-Destructive Determination of Rancidity Levels in Butter Cookies by Multi-Spectral Imaging. J. Sci. Food Agric. 2016, 96, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.M.; Ross, M.; Motoi, L. Bran in Bread: Effects of Particle Size and Level of Wheat and Oat Bran on Mixing, Proving and Baking. In Bubbles in Food 2: Novelty, Health and Luxury; Campbell, G.M., Scanlon, M.G., Pyle, D.L., Eds.; AACC International, Inc.: Eagan, MN, USA, 2008; pp. 337–354. [Google Scholar]
- Chakraborty, P.; Witt, T.; Harris, D.; Ashton, J.; Stokes, J.R.; Smyth, H.E. Texture and Mouthfeel Perceptions of a Model Beverage System Containing Soluble and Insoluble Oat Bran Fibres. Food Res. Int. 2019, 120, 62–72. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Carbohydrate Analysis. In Food Analysis; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 147–177. [Google Scholar]
- Cichero, J.A.Y. Adjustment of Food Textural Properties for Elderly Patients. J. Texture Stud. 2016, 47, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Spence, C. Just How Much of What We Taste Derives from the Sense of Smell? Flavour 2015, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Badawy, W. Utilization of Eggshells By-Product as a Mineral Source for Fortification of Bread Strips. J. Food Dairy Sci. 2017, 8, 455–459. [Google Scholar] [CrossRef]
- Platon, N.; Arus, V.A.; Georgescu, A.M.; Nistor, I.D.; Barsan, N. White Bread Fortified with Calcium from Eggshell Powder. Rev. Chim. 2020, 71, 299–306. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, J.; Singh, V. Effect of Encapsulated Vitamin E on Physical, Storage and Retention Parameters in Cookies. J. Food Sci. Technol. 2020, 57, 3509–3517. [Google Scholar] [CrossRef]
- Khoshakhlagh, K.; Hamdami, N.; Shahedi, M.; Le-Bail, A. Quality and Microbial Characteristics of Part-Baked Sangak Bread Packaged in Modified Atmosphere during Storage. J. Cereal Sci. 2014, 60, 42–47. [Google Scholar] [CrossRef]






| Ingredients | Weight (g) | |
|---|---|---|
| Gingerbread | ||
| 0% ESP | 3% ESP | |
| Mixture 1 | ||
| Milk 3.2% fat | 45.00 | 45.00 |
| Unrefined cane sugar | 5.00 | 5.00 |
| Dry yeast | 2.00 | 2.00 |
| Mixture 2 | ||
| Wheat flour type 650 | 140.00 | 140.00 |
| Oat bran | 35.00 | 35.00 |
| Unrefined cane sugar | 30.00 | 30.00 |
| Salt | 2.00 | 2.00 |
| Honey | 21.00 | 21.00 |
| Egg | 50.00 | 50.00 |
| Baking soda | 2.00 | 2.00 |
| Gingerbread spice mix | 20.00 | 20.00 |
| Butter 82% fat | 19.00 | 19.00 |
| Eggshell powder * | 0.00 | 4.20 |
| Mixture 3 (green tea-chocolate topping) | ||
| White chocolate | 68.00 | 68.00 |
| Green tea powder ** | 3.40 | 3.40 |
| Nutrient (%) | Sample | |
|---|---|---|
| 0% ESP | 3% ESP | |
| Protein | 8.67 ± 0.07 a | 8.76 ± 0.08 a |
| Lipid | 12.19 ± 0.05 a | 12.30 ± 0.09 a |
| Carbohydrate | 61.76 ± 0.10 b | 61.34 ± 0.11 a |
| Ash | 1.46 ± 0.01 a | 3.40 ± 0.05 b |
| Moisture | 15.92 ± 0.11 b | 14.19 ± 0.02 a |
| Energy value (kcal/100 g) | 391.43 | 391.10 |
| Mineral (mg/100 g) | ||
| Calcium | 86.71 ± 1.00 a | 729.42 ± 31.95 b |
| Dietary Fiber (%) | Sample | |
|---|---|---|
| 0% ESP | 3% ESP | |
| NDF | 4.73 ± 0.30 a | 7.09 ± 0.62 b |
| ADF | 2.74 ± 0.20 a | 3.41 ± 0.41 a |
| ADL | 1.19 ± 0.24 a | 1.29 ± 0.26 a |
| ADC | 1.55 ± 0.44 a | 2.12 ± 0.21 a |
| Hemicellulose | 1.99 ± 0.44 a | 3.68 ± 0.89 b |
| SDF | 3.73 ± 0.14 a | 7.13 ± 1.30 b |
| IDF | 1.33 ± 0.25 a | 3.27 ± 0.15 b |
| TDF | 5.06 ± 0.24 a | 10.41 ± 1.43 b |
| Assay | Storage Time (month) | 0% ESP | 3% ESP | ||
|---|---|---|---|---|---|
| TPC | (mg GAE/100 g) | 0 | 215.59 ± 2.69 cA | 214.01 ± 1.18 cA | |
| 1 | 204.75 ± 2.51 bA | 204.67 ± 0.99 bA | |||
| 2 | 198.47 ± 2.06 aA | 196.74 ± 0.58 aA | |||
| ABTS | (mg TE/100 g) | 0 | 453.79 ± 1.18 bA | 448.82 ± 5.11 bA | |
| 1 | 402.73 ± 1.19 aA | 397.40 ± 6.23 aA | |||
| 2 | 396.40 ± 13.66 aA | 388.83 ± 12.48 aA | |||
| DPPH | 0 | 388.13 ± 5.06 cB | 370.44 ± 1.78 cA | ||
| 1 | 372.13 ± 1.23 bB | 354.94 ± 6.43 bA | |||
| 2 | 354.93 ± 5.69 aB | 340.98 ± 3.38 aA | |||
| ORACFL | 0 | 1224.43 ± 39.63 bA | 1194.88 ± 66.1 bA | ||
| 1 | 1003.80 ± 20.36 aA | 1062.21 ± 21.67 abA | |||
| 2 | 996.73 ± 28.43 aA | 1046.38 ± 31.6 aA | |||
| ACW | 0 | 305.49 ± 18.19 bB | 244.99 ± 0.54 bA | ||
| 1 | 219.95 ± 11.25 aA | 222.68 ± 0.36 aA | |||
| 2 | 216.34 ± 1.12 aA | 214.36 ± 4.39 aA | |||
| PCL | ACL | 0 | 256.41 ± 6.63 aA | 289.40 ± 4.35 aB | |
| 1 | 261.19 ± 9.31 aA | 294.61 ± 8.30 aB | |||
| 2 | 287.86 ± 2.82 bA | 302.70 ± 2.86 aB | |||
| IAC | 0 | 561.90 ± 16.15 bA | 536.38 ± 4.30 aA | ||
| 1 | 481.14 ± 14.69 aA | 517.59 ± 12.07 aA | |||
| 2 | 505.56 ± 2.86 aA | 516.89 ± 0.37 aB | |||
| Samples | TPC with | Pearson’s r | Sig. (2-Tailed) |
|---|---|---|---|
| 0% ESP | ABTS | 0.870 | p < 0.01 |
| DPPH | 0.894 | p < 0.01 | |
| ORACFL | 0.923 | p < 0.01 | |
| PCL-ACW | 0.888 | p < 0.01 | |
| PCL-ACL | −0.816 | p < 0.01 | |
| PCL-IAC | 0.707 | p < 0.05 | |
| 3% ESP | ABTS | 0.901 | p < 0.01 |
| DPPH | 0.958 | p < 0.01 | |
| ORACFL | 0.820 | p < 0.01 | |
| PCL-ACW | 0.963 | p < 0.01 | |
| PCL-ACL | −0.726 | p < 0.05 | |
| PCL-IAC | 0.814 | p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnold, M.; Rajagukguk, Y.V.; Sidor, A.; Kulczyński, B.; Brzozowska, A.; Suliburska, J.; Wawrzyniak, N.; Gramza-Michałowska, A. Innovative Application of Chicken Eggshell Calcium to Improve the Functional Value of Gingerbread. Int. J. Environ. Res. Public Health 2022, 19, 4195. https://doi.org/10.3390/ijerph19074195
Arnold M, Rajagukguk YV, Sidor A, Kulczyński B, Brzozowska A, Suliburska J, Wawrzyniak N, Gramza-Michałowska A. Innovative Application of Chicken Eggshell Calcium to Improve the Functional Value of Gingerbread. International Journal of Environmental Research and Public Health. 2022; 19(7):4195. https://doi.org/10.3390/ijerph19074195
Chicago/Turabian StyleArnold, Marcellus, Yolanda Victoria Rajagukguk, Andrzej Sidor, Bartosz Kulczyński, Anna Brzozowska, Joanna Suliburska, Natalia Wawrzyniak, and Anna Gramza-Michałowska. 2022. "Innovative Application of Chicken Eggshell Calcium to Improve the Functional Value of Gingerbread" International Journal of Environmental Research and Public Health 19, no. 7: 4195. https://doi.org/10.3390/ijerph19074195