Distribution, Removal, and Risk Assessment of Pharmaceuticals and Their Metabolites in Five Sewage Plants
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Methods
2.2. Sample Collection
2.3. Sample Preparation
2.4. Analytical Protocol
2.5. Quality Assurance and Quality Control
2.6. Calculation of Mass Loads and Removal Efficiency
3. Results and Discussion
3.1. Occurrence of PPCPs at Different Sewage Treatment Plants
3.1.1. Distribution of PPCPs in the Influent of Sewage Treatment Plants
3.1.2. Distribution of PPCPs in the Effluent of Sewage Treatment Plants
3.1.3. Distribution of PPCPs in Sludge and Suspended Particles
3.2. Mass Loads and Mass Balance Analysis
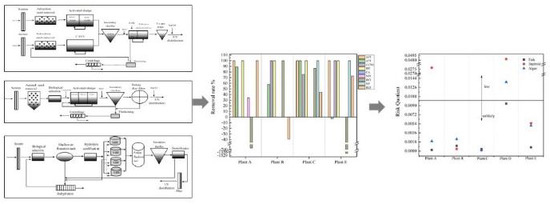
3.3. Removal Efficiencies of Different Sewage Treatment Plants
3.3.1. Removal Rate in Sewage Treatment Plant
3.3.2. Removal Efficiency in the Biological Treatment Unit
3.3.3. Removal Efficiency of the Precipitation, Filtration, and Ultraviolet Disinfection Process
4. Risk Assessment of PPCPs in Effluent
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Houtman, C.J.; Kroesbergen, J.; Lekkerkerker-Teunissen, K.; van der Hoek, J.P. Human health risk assessment of the mixture of pharmaceuticals in Dutch drinking water and its sources based on frequent monitoring data. Sci. Total Environ. 2014, 496, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.M.; Barber, L.B.; Duris, J.W.; Foreman, W.T.; Furlong, E.T.; Hubbard, L.E.; Hutchinson, K.J.; Keefe, S.H.; Kolpin, D.W. Riverbank filtration potential of pharmaceuticals in a wastewater-impacted stream. Environ. Pollut. 2014, 193, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Zhenhua, Y.; Guanghua, L.; Qiuxia, Y.; Jianchao, L. Long-term effects of antibiotics, norfloxacin, and sulfamethoxazole, in a partial life-cycle study with zebrafish (Danio rerio): Effects on growth, development, and reproduction. Environ. Sci. Pollut. Res. Int. 2016, 23, 18222–18228. [Google Scholar]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef]
- Ziylan, A.; Ince, N.H. The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: Treatability by conventional and non-conventional processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. J. Hazard. Mater. 2010, 179, 804–817. [Google Scholar] [CrossRef]
- Weining, W.; Xi, C.; Haimei, J. Simultaneous determination of multiple antibiotic residues in livestock manure by solid phase Extraction-Ultra performance liquid chromatography tandem mass spectrometry. Chin. J. Anal. Chem. 2013, 41, 993–999. [Google Scholar]
- Ashfaq, M.; Li, Y.; Rehman, M.S.U.; Zubair, M.; Mustafa, G.; Nazar, M.F.; Yu, C.; Sun, Q. Occurrence, spatial variation and risk assessment of pharmaceuticals and personal care products in urban wastewater, canal surface water, and their sediments: A case study of Lahore, Pakistan. Sci. Total Environ. 2019, 688, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Ekpeghere, K.I.; Sim, W.; Lee, H.; Oh, J. Occurrence and distribution of carbamazepine, nicotine, estrogenic compounds, and their transformation products in wastewater from various treatment plants and the aquatic environment. Sci. Total Environ. 2018, 640–641, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Kim, M.; Shah, A.; Alaee, M.; Smyth, S.A. Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci. Total Environ. 2014, 473–474, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Östman, M.; Fick, J.; Tysklind, M. Detailed mass flows and removal efficiencies for biocides and antibiotics in Swedish sewage treatment plants. Sci. Total Environ. 2018, 640–641, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Wu, R.; Lam, J.C.W.; Zhang, K.; Lam, P.K.S. Seasonal occurrence and fate of chiral pharmaceuticals in different sewage treatment systems in Hong Kong: Mass balance, enantiomeric profiling, and risk assessment. Water Res. 2019, 149, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.; Watts, C.; Boucard, T. Chronic aquatic environmental risks from exposure to human pharmaceuticals. Sci. Total Environ. 2006, 367, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Al-Odaini, N.A.; Zakaria, M.P.; Yaziz, M.I.; Surif, S.; Abdulghani, M. The occurrence of human pharmaceuticals in wastewater effluents and surface water of Langat River and its tributaries, Malaysia. Int. J. Environ. Anal. Chem. 2013, 93, 245–264. [Google Scholar] [CrossRef]
- Koutsouba, V.; Heberer, T.; Fuhrmann, B.; Schmidt-Baumler, K.; Tsipi, D.; Hiskia, A. Determination of polar pharmaceuticals in sewage water of Greece by gas chromatography–mass spectrometry. Chemosphere 2003, 51, 69–75. [Google Scholar] [CrossRef]
- Wiegel, S.; Aulinger, A.; Brockmeyer, R.; Harms, H.; Löffler, J.; Reincke, H.; Schmidt, R.; Stachel, B.; von Tümpling, W.; Wanke, A. Pharmaceuticals in the river Elbe and its tributaries. Chemosphere 2004, 57, 107–126. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci. Total Environ. 2012, 430, 109–118. [Google Scholar] [CrossRef]
- Lindqvist, N.; Tuhkanen, T.; Kronberg, L. Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters. Water Res. 2005, 39, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, L.Y.; Li, L.S.; Xu, L. Photodegradation kinetics, transformation, and toxicity prediction of ketoprofen, carprofen, and diclofenac acid in aqueous solutions. Environ. Toxicol. Chem. 2017, 36, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Lin, C.; Tung, H.; Chary, N.S. Potential for biodegradation and sorption of acetaminophen, caffeine, propranolol and acebutolol in lab-scale aqueous environments. J. Hazard. Mater. 2010, 183, 242–250. [Google Scholar] [CrossRef]
- Rodil, R.; Quintana, J.B.; Cela, R. Transformation of phenazone-type drugs during chlorination. Water Res. 2012, 46, 2457–2468. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Davididou, K.; Monteagudo, J.M.; Chatzisymeon, E.; Durán, A.; Expósito, A.J. Degradation and mineralization of antipyrine by UV-A LED photo-Fenton reaction intensified by ferrioxalate with addition of persulfate. Sep. Purif. Technol. 2017, 172, 227–235. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Zhang, Y.; Sui, M.; Deng, J.; Zhou, S. Degradation of antipyrine by UV, UV/H2O2 and UV/PS. J. Hazard. Mater. 2013, 260, 1008–1016. [Google Scholar] [CrossRef]
- Bahlmann, A.; Brack, W.; Schneider, R.J.; Krauss, M. Carbamazepine and its metabolites in wastewater: Analytical pitfalls and occurrence in Germany and Portugal. Water Res 2014, 57, 104–114. [Google Scholar] [CrossRef]
- Boyd, G.R.; Zhang, S.; Grimm, D.A. Naproxen removal from water by chlorination and biofilm processes. Water Res. 2004, 39, 668–676. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gomez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Sci. Total Environ. 2008, 407, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Feng, L.; Fu, J.; Li, X.; Li, P.; Zhang, Y. The fate of typical pharmaceuticals in wastewater treatment plants of Xi’an city in China. J. Environ. Chem. Eng. 2015, 3, 2203–2211. [Google Scholar] [CrossRef]
- Spongberg, A.L.; Witter, J.D. Pharmaceutical compounds in the wastewater process stream in Northwest Ohio. Sci. Total Environ. 2008, 397, 148–157. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- Gallardo-Altamirano, M.J.; Maza-Márquez, P.; Peña-Herrera, J.M.; Rodelas, B.; Osorio, F.; Pozo, C. Removal of anti-inflammatory/analgesic pharmaceuticals from urban wastewater in a pilot-scale A2O system: Linking performance and microbial population dynamics to operating variables. Sci. Total Environ. 2018, 643, 1481–1492. [Google Scholar] [CrossRef]
- Escher, B.I.; Baumgartner, R.; Koller, M.; Treyer, K.; Lienert, J.; McArdell, C.S. Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res. 2011, 45, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Grung, M.; Källqvist, T.; Sakshaug, S.; Skurtveit, S.; Thomas, K.V. Environmental assessment of Norwegian priority pharmaceuticals based on the EMEA guideline. Ecotoxicol. Environ. Saf. 2008, 71, 328–340. [Google Scholar] [CrossRef]
- Stuer-Lauridsen, F.; Birkved, M.; Hansen, L.P.; Lutzhoft, H.C.; Halling-Sorensen, B. Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use. Chemosphere 2000, 40, 783–793. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Lee, D.S.; Kim, Y. Risk assessment of human antibiotics in Korean aquatic environment. Environ. Toxicol. Pharmacol. 2008, 26, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. LC-MS analysis and environmental risk of lipid regulators. Anal. Bioanal. Chem. 2007, 387, 1269–1285. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, H. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003, 144, 383–395. [Google Scholar] [CrossRef]
- Archana, G.; Dhodapkar, R.; Kumar, A. Ecotoxicological risk assessment and seasonal variation of some pharmaceuticals and personal care products in the sewage treatment plant and surface water bodies (lakes). Environ. Monit. Assess. 2017, 189, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Brezina, E.; Prasse, C.; Meyer, J.; Mückter, H.; Ternes, T.A. Investigation and risk evaluation of the occurrence of carbamazepine, oxcarbazepine, their human metabolites and transformation products in the urban water cycle. Environ. Pollut. 2017, 225, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Meng, X.; Wen, Z.; Ke, R.; Chen, L. Multi-phase partitioning, ecological risk and fate of acidic pharmaceuticals in a wastewater receiving river: The role of colloids. Sci. Total Environ. 2013, 447, 267–273. [Google Scholar] [CrossRef]





| Compounds | Molecular Formula | Physicochemical Properties | Use |
|---|---|---|---|
| Antipyrine ATP CAS:60-80-0 |  | MW: 188.23 Log Kow: 0.59 WS: 1000 g/L | Antipyretic, analgesic |
| Naproxen NPX CAS:22204-53-1 |  | MW: 230.26 Log Kow: 2.5 | Antipyretic, analgesic |
| Acetaminophen ACE * CAS:130-90-2 |  | MW: 151.16 Log Kow: 0.27 WS: 14 g/L | Antipyretic, analgesic |
| 4-Chlorobenzoic acid 4-CBA * CAS:74-11-3 |  | MW: 156.57 Log Kow: 2.52 | Hypolipidemic |
| Ibuprofen IPF CAS:15687-27-1 |  | MW: 206.28 Log Kow: 3.79 | Anti-inflammatory |
| Clofibric acid CA CAS:882-09-7 |  | MW: 214.64 Log Kow: 2.84 | Hypolipidemic |
| Nifedipine NP CAS:21829-25-4 |  | MW: 346.34 Log Kow: 2.50 | Antihypertensive |
| Bezafibrate BZB CAS:41859-67-0 |  | MW: 361.83 Log Kow: 4.25 | Hypolipidemic |
| Diclofenac DCF CAS:15307-86-5 |  | MW: 296.15 Log Kow: 4.02 | Anti-inflammatory, analgesic |
| PPCPs | ACE | ATP | 4-CBA | IPF | CA | NPX | DCF | NP | BZB | |
|---|---|---|---|---|---|---|---|---|---|---|
| Plant A | Influent (ng/L) | 511.3 ± 35.5 | 0.9 ± 0.1 | - | 21.8 ± 2.5 | - | 3.8 ± 0.8 | 2.9 ± 0.4 | - | 0.65 ± 0.07 |
| Effluent (ng/L) | - | 0.1 ± 0.0 | - | - | 2.6 ± 0.5 | 2.5 ± 0.6 | 22.6 ± 1.8 | - | - | |
| Sludge (ng/g) | - | - | 0.9 ± 0.1 | - | - | - | - | - | - | |
| Particulate (ng/g) | - | - | 14.5 ± 9.5 | - | 3.0 ± 0.3 | - | - | - | - | |
| Plant B | Influent (ng/L) | - | 5.8 ± 0.3 | - | - | - | - | 5.7 ± 1.3 | - | 0.8 ± 0.1 |
| Effluent (ng/L) | - | 2.4 ± 0.1 | - | - | - | - | - | - | 1.1 ± 0.1 | |
| Sludge (ng/g) | - | - | 2.3 ± 1.9 | - | - | - | 3.2 ± 0.6 | - | - | |
| Particulate (ng/g) | - | - | 29.1 ± 1.2 | - | - | - | 14.6 ± 1.9 | - | - | |
| Plant C | Influent (ng/L) | 208 ± 23.9 | 4.1 ± 0.9 | 23.9 ± 5.6 | 49.6 ± 2.2 | 2.2 ± 0.1 | - | 9.2 ± 1.5 | - | 4.1 ± 0.6 |
| Effluent (ng/L) | - | - | 5.8 ± 1.6 | - | - | - | 1.2 ± 0.5 | - | 2.3 ± 0.1 | |
| Sludge (ng/g) | - | - | 1.1 ± 0.1 | - | 3.3 ± 0.6 | - | - | - | - | |
| Particulate (ng/g) | - | - | 12.8 ± 4.2 | - | - | - | 10.0 ± 0.8 | - | - | |
| Plant D | Influent (ng/L) | - | 1.2 ± 0.1 | 88.0 ± 4.7 | - | - | 4.3 ± 2.4 | 61.2 ± 2.9 | - | 2.2 ± 0.3 |
| Effluent (ng/L) | 360.2 ± 35.1 | 1.9 ± 0.2 | 4.1 ± 0.7 | 38.6 ± 2.8 | - | - | 4.1 ± 0.3 | 13.7 ± 2.1 | - | |
| Particulate (ng/g) | - | - | 11.2 ± 2.1 | - | - | 80.5 ± 23.8 | 6.6 ± 0.8 | - | - | |
| Plant E | Influent(ng/L) | 510.8 ± 25.3 | 1.1 ± 0.1 | 16.4 ± 0 | 23.3 ± 2.2 | - | - | 3.7 ± 1.1 | 19.8 ± 20.9 | 2.8 ± 0.8 |
| Effluent (ng/L) | - | 1.2 ± 0.1 | - | - | 0.3 ± 0.1 | - | 55 ± 0.8 | - | 0.8 ± 0.5 | |
| Sludge (ng/g) | - | - | 4.4 ± 0.5 | - | 1.3 ± 0.1 | - | 4.2 ± 0.8 | - | - | |
| Particulate (ng/g) | - | - | 35.5 ± 9.5 | - | - | - | 5.2 ± 0.8 | - | - | |
| PPCPs | ACE | ATP | 4-CBA | IPF | CA | NPX | DCF | NP | BZB | |
|---|---|---|---|---|---|---|---|---|---|---|
| Plant A | Influent (mg/d) | 15,696.9 | 27.6 | - | 669.3 | - | 116.7 | 89.0 | - | 20.0 |
| Effluent (mg/d) | - | 3.1 | - | - | 79.8 | 76.8 | 693.8 | - | - | |
| Sludge (mg/d) | - | - | 15.4 | - | - | - | - | - | - | |
| Mass loss (mg/L) | 15,696.9 | 24.6 | −15.4 | 669.3 | −79.8 | 39.9 | −604.8 | - | 20.0 | |
| Total mass loss | 100% | 89% | - | 100% | - | 34% | −679% | - | 100% | |
| Plant B | Influent (mg/d) | - | 87.0 | - | - | - | - | 85.5 | - | 12.0 |
| Effluent (mg/d) | - | 36.0 | - | - | - | - | - | - | 16.5 | |
| Sludge (mg/d) | - | - | 14.4 | - | - | - | 20.0 | - | - | |
| Mass loss (mg/L) | - | 51.0 | −14.4 | - | - | - | 65.5 | - | −4.5 | |
| Total mass loss | - | 59% | - | - | - | - | 77% | - | −38% | |
| Plant C | Influent (mg/d) | 14,887.0 | 293.4 | 1710.6 | 3550.0 | 157.5 | - | 658.5 | - | 293.4 |
| Effluent (mg/d) | - | - | 415.1 | - | - | - | 85.9 | - | 164.6 | |
| Sludge (mg/d) | - | - | 54.2 | - | 162.5 | - | - | - | - | |
| Mass loss (mg/L) | 14,887.0 | 293.4 | 1241.3 | 3550.0 | −5.1 | - | 572.6 | - | 128.8 | |
| Total mass loss | 100% | 100% | 73% | 100% | −3% | - | 87% | - | 44% | |
| Plant E | Influent (mg/d) | 51,080.0 | 110.0 | 1640.0 | 2330.0 | - | - | 370.0 | 1980.0 | 280.0 |
| Effluent (mg/d) | - | 120.0 | - | - | - | - | 5500.0 | - | 80.0 | |
| Sludge (mg/d) | - | - | 298.1 | - | 88.1 | - | 284.6 | - | - | |
| Mass loss (mg/L) | 51,080.0 | −10.0 | 1341.9 | 2330.0 | −88.1 | - | −5414.6 | 1980.0 | 200.0 | |
| Total mass loss | 100% | −9% | 82% | 100% | - | - | −1463% | 100% | 71% |
| STP | Treat Process | ACE | ATP | 4-CBA | IPF | CA | NPX | DCF | NP | BZB |
|---|---|---|---|---|---|---|---|---|---|---|
| Plant A | Anaerobic | −11% | −89% | - | −31% | - | −273% | −130% | - | −264% |
| Hypoxic | 100% | −134% | - | 100% | - | 30% | −1442% | - | 70% | |
| Aerobic | - | 1% | - | - | 23% | 21% | 8% | - | −29% | |
| Secondary clarifier | - | 2% | - | - | −4% | −4% | 10% | - | −4% | |
| Inclined plate clarifier | - | 17% | - | - | 28% | −11% | 72% | - | 236% | |
| Effluent | 100% | 97% | - | 100% | −24% | 73% | 6% | 100% | 100% | |
| Plant B | Chemical reaction cell | - | −3% | - | - | - | - | 61% | - | −61% |
| Air flotation tank | - | −1% | - | 100% | - | - | −123% | - | 32% | |
| SBR | - | 18% | - | - | - | - | −169% | - | −124% | |
| Fenton | - | 47% | - | - | - | - | 69% | - | 50% | |
| Triple settling tank | - | 15% | 100% | - | - | - | −11% | - | 7% | |
| Effluent | - | −11% | - | - | - | - | 100% | - | −23% | |
| Plant C | Anaerobic | −43% | −11% | −5% | −200% | −25% | - | −627% | - | 34% |
| Hypoxic | 96% | −39% | 62% | 59% | 22% | - | −18% | 20% | 2% | |
| Aerobic | 100% | −55% | 100% | 100% | 0% | - | −4% | 100% | −5% | |
| Secondary clarifier | - | 30% | - | - | −5% | - | −4% | - | 18% | |
| Inclined plate clarifier | - | −20% | 2% | - | −16% | - | −9% | - | 3% | |
| Effluent | - | 100% | 33% | - | 16% | - | 99% | - | −3% | |
| Plant D | Anaerobic/Hypoxic | - | −183% | −374% | - | - | 100% | 37% | - | 19% |
| Aeration | 100% | −79% | 68% | −9% | - | - | −92% | 7% | −33% | |
| Grate | - | −129% | 13% | 79% | - | - | −12% | 100% | 21% | |
| Anaerobic | 98% | −36% | −397% | −58% | - | 100% | −1112% | - | 45% | |
| Aerobic | 100% | −9% | 23% | 100% | - | - | −20% | - | 100% | |
| Secondary clarifier | - | −40% | 55% | - | - | −16% | 0% | 100% | - | |
| Effluent | - | 0% | - | - | - | 8% | - | - | - | |
| Plant E | Anaerobic | 37% | −24% | −187% | −283% | - | - | −1228% | −236% | 4% |
| Hypoxic | 100% | −50% | 100% | 100% | - | - | −34% | −9% | 85% | |
| Aerobic | - | 2% | - | - | - | - | −1% | 77% | −88% | |
| Secondary clarifier | - | 3% | 13% | - | - | - | −1% | 100% | 2% | |
| Sand filter | - | −2% | 100% | - | - | - | 20% | - | −18% | |
| Effluent | - | 43% | - | - | - | - | −4% | - | 12% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Niu, X.; Yao, C.; Yang, W.; Lu, G. Distribution, Removal, and Risk Assessment of Pharmaceuticals and Their Metabolites in Five Sewage Plants. Int. J. Environ. Res. Public Health 2019, 16, 4729. https://doi.org/10.3390/ijerph16234729
Li Y, Niu X, Yao C, Yang W, Lu G. Distribution, Removal, and Risk Assessment of Pharmaceuticals and Their Metabolites in Five Sewage Plants. International Journal of Environmental Research and Public Health. 2019; 16(23):4729. https://doi.org/10.3390/ijerph16234729
Chicago/Turabian StyleLi, Ying, Xiangming Niu, Chi Yao, Wen Yang, and Guanghua Lu. 2019. "Distribution, Removal, and Risk Assessment of Pharmaceuticals and Their Metabolites in Five Sewage Plants" International Journal of Environmental Research and Public Health 16, no. 23: 4729. https://doi.org/10.3390/ijerph16234729