Manipulation of the Phenolic Quality of Assam Green Tea through Thermal Regulation and Utilization of Microwave and Ultrasonic Extraction Techniques
Abstract
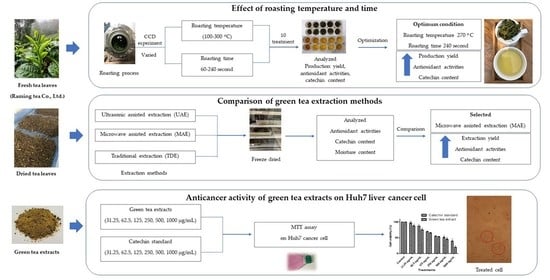
:1. Introduction
2. Materials and Methods
2.1. Preparation of Roasted Green Tea
2.2. Green Tea Extraction
2.2.1. Microwave-Assisted Extraction (MAE)
2.2.2. Ultrasound-Assisted Extraction (UAE)
2.2.3. Traditional Extraction (TDE)
2.3. Production and Extraction Yield
2.4. Physical and Chemical Analysis
2.5. Total Phenolic Compound (TPC) Content
2.6. 2,2-Diphenyl-l-picryl-hydrazyl (DPPH) Assay
2.7. Ferric Reducing/Antioxidant Power (FRAP) Assay
2.8. ABTS Decolorization Assay
2.9. Quantification of Catechin Content
2.10. Sensory Evaluation of Roasted Green Tea
2.11. Human Cell Culture
2.12. Cell Viability Assay
2.13. Statistical Analysis
3. Results and Discussions
3.1. Effect of Roasting Temperature and Time on Green Tea Production Yield
3.2. Effect of Roasting Temperature and Time on Green Tea Antioxidant Activity
3.3. Effect of Roasting Temperature and Time on Green Tea Catechin Content
3.4. Effect of Roasting Temperature and Time on Green Tea Sensory Acceptance
3.5. Optimization of the Roasting Process
3.6. Effect of Extraction Method on Catechin Content
3.6.1. Effect of Ultrasonic Assisted Extraction (UAE)
3.6.2. Effect of Microwave-Assisted Extraction (MAE)
3.6.3. Effect of Traditional Extraction (TDE)
3.6.4. Comparison of UAE, MAE and TDE
3.7. Anticancer Activity of Green Tea Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TR | treatment |
| CCD | central composite design |
| Yield after roasting | production yield after roasting: calculated as the ratio of weight of fresh green tea leaves before processing to that of roasted green tea leaves |
| Yield after drying | production yield after drying: calculated as the ratio of weight of fresh green tea leaves before processing to that of dried green tea leaves |
| MC | moisture content |
| aw | water activity |
| DW | dry weight |
| TPC | total phenolic content |
| GAE | gallic acid equivalent |
| DPPH | 2,2-diphenyl-l-picryl-hydrazyl |
| FRAP | ferric reducing antioxidant power |
| ABTS | 2,2′-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid |
| MAE | microwave-assisted extraction |
| UAE | ultrasonic-assisted extraction |
| TDE | traditional extraction |
| C | catechin |
| EGCG | epigallocatechin gallate |
| EGC | epigallocatechin |
| ECG | epicatechin gallate |
| EC | epicatechin |
| PPO | polyphenol oxidase |
References
- Moderno, P.M.; Carvalho, M.; Silva, B.M. Recent patents on Camellia sinensis: Source of health promoting compounds. Recent Pat. Food Nutr. Agric. 2009, 1, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-M.; Sun, C.; Butler, L.M. Tea and cancer prevention: Epidemiological studies. Pharmacol. Res. 2011, 64, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, M.; Jerónimo, C.; Valentão, P.; Andrade, P.B.; Silva, B.M. Green tea: A promising anticancer agent for renal cell carcinoma. Food Chem. 2010, 122, 49–54. [Google Scholar] [CrossRef]
- Zhu, Y.-M.; Dong, J.-J.; Jin, J.; Liu, J.-H.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R.; Ye, J.-H. Roasting process shaping the chemical profile of roasted green tea and the association with aroma features. Food Chem. 2021, 353, 129428. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Han, J.-A.; Lim, S.-T.; Cho, D.-H. Effects of germination and roasting on physicochemical and sensory characteristics of brown rice for tea infusion. Food Chem. 2021, 350, 129240. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Li, P.; Yang, J.; Wang, F.; Kim, E.; Wu, Y.; He, P.; Li, B.; Tu, Y. Chemical characterization of Wuyi rock tea with different roasting degrees and their discrimination based on volatile profiles. RSC Adv. 2021, 11, 12074–12085. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Ji, W.-B.; Yu, P.; Chen, J.-X.; Wang, F.; Yin, J.-F. Effect of extraction methods on the chemical components and taste quality of green tea extract. Food Chem. 2018, 248, 146–154. [Google Scholar] [CrossRef]
- Das, P.R.; Eun, J.-B. A comparative study of ultra-sonication and agitation extraction techniques on bioactive metabolites of green tea extract. Food Chem. 2018, 253, 22–29. [Google Scholar] [CrossRef]
- Wang, L.; Qin, P.; Hu, Y. Study on the microwave-assisted extraction of polyphenols from tea. Front. Chem. Eng. China 2010, 4, 307–313. [Google Scholar] [CrossRef]
- Lee, L.-S.; Lee, N.; Kim, Y.H.; Lee, C.-H.; Hong, S.P.; Jeon, Y.-W.; Kim, Y.-E. Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 2013, 18, 13530–13545. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; 33.2.11 (method 991.20); 33.2.27A (method 2000.18); AOAC International: Gaitherburg, MD, USA, 2006. [Google Scholar]
- Izzreen, N.Q.; Mohd Fadzelly, A. Phytochemicals and antioxidant properties of different parts of Camellia sinensis leaves from Sabah Tea Plantation in Sabah, Malaysia. Int. Food Res. J. 2013, 20, 307. [Google Scholar]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phyther. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. WJG 2013, 19, 7726. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Levin, C.E.; Lee, S.; Kozukue, N. Stability of green tea catechins in commercial tea leaves during storage for 6 months. J. Food Sci. 2009, 74, H47–H51. [Google Scholar] [CrossRef] [PubMed]
- Saloko, S.; Sulastri, Y.; Murad, M.; Rinjani, M.A. The effects of temperature and roasting time on the quality of ground Robusta coffee (Coffea rabusta) using Gene Café roaster. In Proceedings of the AIP Conference Proceedings, 23 December 2019; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2199, p. 60001. [Google Scholar]
- Şahin, H.; Topuz, A.; Pischetsrieder, M.; Özdemir, F. Effect of roasting process on phenolic, antioxidant and browning properties of carob powder. Eur. Food Res. Technol. 2009, 230, 155–161. [Google Scholar] [CrossRef]
- Lund, M.; Ray, C. Control of maillard Reactions in foods: Strategies and chemical mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [Green Version]
- Khokhar, S.; Magnusdottir, S.G.M. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef]
- Donlao, N.; Ogawa, Y. The influence of processing conditions on catechin, caffeine and chlorophyll contents of green tea (Camelia sinensis) leaves and infusions. LWT 2019, 116, 108567. [Google Scholar] [CrossRef]
- Chen, C.-N.; Liang, C.-M.; Lai, J.-R.; Tsai, Y.-J.; Tsay, J.-S.; Lin, J.-K. Capillary electrophoretic determination of theanine, caffeine, and catechins in fresh tea leaves and oolong tea and their effects on rat neurosphere adhesion and migration. J. Agric. Food Chem. 2003, 51, 7495–7503. [Google Scholar] [CrossRef] [PubMed]
- Gramza, A.; Khokhar, S.; Yoko, S.; Gliszczynska-Swiglo, A.; Hes, M.; Korczak, J. Antioxidant activity of tea extracts in lipids and correlation with polyphenol content. Eur. J. Lipid Sci. Technol. 2006, 108, 351–362. [Google Scholar] [CrossRef]
- Ünal, M.Ü.; Taş, C.; Şener, A. Determination of biochemical properties of polyphenol oxidase from domat olives. Gida 2011, 36, 185–192. [Google Scholar]
- Ünal, M.Ü.; Yabaci, S.N.; Şener, A. Extraction, partial purification and characterisation of polyphenol oxidase from tea leaf (Camellia sinesis). Gida 2011, 36, 137–144. [Google Scholar]
- Baruah, A.M. Fermentation characteristics of some Assamica clones and process optimization of black tea manufacturing. J. Agric. Food Chem. 2003, 51, 6578–6588. [Google Scholar] [CrossRef] [PubMed]
- Gejima, Y.; Nagata, M. Basic study on Kamairicha tea leaves quality judgment system. In Proceedings of the 2000 ASAE Annual International Meeting, Milwaukee, WI, USA, 9–12 July 2000; pp. 1–10. [Google Scholar]
- Nguyen, H.T.H.; Ong, L.; Kentish, S.E.; Gras, S.L. Homogenisation improves the microstructure, syneresis and rheological properties of buffalo yoghurt. Int. Dairy J. 2015, 46, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Cloughley, J.B. The effect of temperature on enzyme activity during the fermentation phase of black tea manufacture. J. Sci. Food Agric. 1980, 31, 920–923. [Google Scholar] [CrossRef]
- Ullah, M.R.; Roy, P.C. Effect of withering on the polyphenol oxidase level in the tea leaf. J. Sci. Food Agric. 1982, 33, 492–495. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. An Int. J. Devoted to Rapid Dissem. Up-to-the-Minute Res. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef]
- Wang, Y.; Kan, Z.; Thompson, H.J.; Ling, T.; Ho, C.-T.; Li, D.; Wan, X. Impact of six typical processing methods on the chemical composition of tea leaves using a single Camellia sinensis cultivar, Longjing 43. J. Agric. Food Chem. 2018, 67, 5423–5436. [Google Scholar] [CrossRef]
- Friedman, M.; Kim, S.; Lee, S.; Han, G.; Han, J.; Lee, K.; Kozukue, N. Distribution of catechins, theaflavins, caffeine, and theobromine in 77 teas consumed in the United States. J. Food Sci. 2005, 70, C550–C559. [Google Scholar] [CrossRef]
- Perva-Uzunalić, A.; Škerget, M.; Knez, Ž.; Weinreich, B.; Otto, F.; Grüner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Hemwimon, S.; Pavasant, P.; Shotipruk, A. Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep. Purif. Technol. 2007, 54, 44–50. [Google Scholar] [CrossRef]
- Grujic, N.; Lepojevic, Z.; Srdjenovic, B.; Vladic, J.; Sudji, J. Effects of different extraction methods and conditions on the phenolic composition of mate tea extracts. Molecules 2012, 17, 2518–2528. [Google Scholar] [CrossRef] [Green Version]
- Chew, K.K.; Khoo, M.Z.; Ng, S.Y.; Thoo, Y.Y.; Aida, W.W.M.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427. [Google Scholar]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhang, M.; Wang, Z. Improved extraction of oil from chickpea under ultrasound in a dynamic system. J. Food Eng. 2010, 98, 13–18. [Google Scholar] [CrossRef]
- Swamy, K.M.; Narayana, K.L. Intensification of leaching process by dual-frequency ultrasound. Ultrason. Sonochem. 2001, 8, 341–346. [Google Scholar] [CrossRef]
- Banerjee, S.; Chatterjee, J. Efficient extraction strategies of tea (Camellia sinensis) biomolecules. J. Food Sci. Technol. 2015, 52, 3158–3168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tu, Z.; Yuan, T.; Wang, H.; Fu, Z.; Wen, Q.; Wang, X. Solvent optimization, antioxidant activity, and chemical characterization of extracts from Artemisia selengnesis Turcz. Ind. Crops Prod. 2014, 56, 223–230. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. Process Intensif. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- John, K.M.M.; Vijayan, D.; Kumar, R.R.; Premkumar, R. Factors influencing the efficiency of extraction of polyphenols from young tea leaves. Asian J. Plant Sci. 2006, 5, 123–126. [Google Scholar]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Yeong, Y.L.; Pang, S.F.; Chong, S.Y.; Gimbun, J. Comparison of microwave and ultrasonic assisted extraction of kaempferol from Cassia alata. Int. J. Eng. Technol. 2018, 7, 84–89. [Google Scholar] [CrossRef]
- Xia, T.; Shi, S.; Wan, X. Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion. J. Food Eng. 2006, 74, 557–560. [Google Scholar] [CrossRef]
- Horžić, D.; Jambrak, A.R.; Belščak-Cvitanović, A.; Komes, D.; Lelas, V. Comparison of conventional and ultrasound assisted extraction techniques of yellow tea and bioactive composition of obtained extracts. Food Bioprocess Technol. 2012, 5, 2858–2870. [Google Scholar] [CrossRef]
- Rha, C.-S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.-O. Antioxidative, anti-inflammatory, and anticancer effects of purified flavonol glycosides and aglycones in green tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Nakachi, K.; Matsuyama, S.; Miyake, S.; Suganuma, M.; Imai, K. Preventive effects of drinking green tea on cancer and cardiovascular disease: Epidemiological evidence for multiple targeting prevention. Biofactors 2000, 13, 49–54. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Chen, F.; Hu, X.; Xu, D.; Cao, Y. Epimerisation and hydrolysis of catechins under ultrasonic treatment. Int. J. Food Sci. Technol. 2021, 56, 312–320. [Google Scholar] [CrossRef]
- Huang, X.; Cao, H.; Guo, Y.; Liu, J.; Sun, Y.; Liu, S.; Lin, J.; Wei, S.; Wu, L. The dynamic change of oolong tea constitutes during enzymatic-catalysed process of manufacturing. Int. J. Food Sci. Technol. 2020, 55, 3604–3612. [Google Scholar] [CrossRef]
- Peng, Y.; Xi, J.; Sun, Y.; Chen, G.; Li, D.; Peng, C.; Wan, X.; Cai, H. Tea components influencing bioavailability of fluoride and potential transport mechanism in the Caco-2 cell line model. Int. J. Food Sci. Technol. 2020, 55, 1792–1799. [Google Scholar] [CrossRef]


 : optimum point.
: optimum point.
 : optimum point.
: optimum point.

| TR b | Temperature (°C) | Time (s) | Yield after Roasting c (%) | Yield after Drying d (%) | MC e (%) | aW | Antioxidant Activities (mg GAE/ g DW f) | Catechin Content (mg/g DW f) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | DPPH | ABTS | FRAP | C | EGCG | EGC | ECG | EC | |||||||
| 1 | 58.5 | 150 | 66.0 | 20.7 | 3.1 | 0.31 | 80.45 ± 6.84 | 28.97 ± 0.56 | 18.34 ± 1.29 | 12.76 ± 0.65 | 7.71 ± 0.48 | 4.54 ± 0.30 | 0.15 ± 0.09 | 3.29 ± 0.86 | 7.97 ± 0.87 |
| 2 | 100.0 | 60 | 68.7 | 20.9 | 3.1 | 0.32 | 124.60 ± 2.39 | 48.14 ± 1.23 | 24.23 ± 2.06 | 17.77 ± 1.71 | 9.51 ± 1.24 | 6.95 ± 0.99 | 1.92 ± 0.25 | 3.63 ± 0.45 | 8.61 ± 0.53 |
| 3 | 100.0 | 240 | 64.0 | 20.6 | 3.2 | 0.30 | 128.83 ± 4.11 | 45.08 ± 3.82 | 23.31 ± 1.34 | 17.07 ± 1.55 | 9.59 ± 1.19 | 7.48 ± 0.87 | 2.18 ± 0.54 | 9.03 ± 1.41 | 10.48 ± 1.28 |
| 4 | 200.0 | 22 | 68.0 | 21.2 | 3.2 | 0.31 | 107.02 ± 5.83 | 33.60 ± 1.65 | 20.02 ± 1.78 | 12.92 ± 0.82 | 8.77 ± 0.40 | 4.88 ± 0.63 | 0.50 ± 0.12 | 2.88 ± 0.21 | 7.81 ± 1.39 |
| 5 | 200.0 | 150 | 66.0 | 20.7 | 3.3 | 0.32 | 154.63 ± 3.34 | 53.56 ± 2.01 | 26.98 ± 2.28 | 21.11 ± 1.12 | 10.11 ± 0.88 | 10.57 ± 0.98 | 3.73 ± 0.43 | 9.92 ± 0.75 | 13.45 ± 1.04 |
| 6 | 200.0 | 150 | 65.5 | 20.4 | 3.2 | 0.30 | 168.28 ± 4.12 | 55.43 ± 1.46 | 27.03 ± 1.77 | 20.78 ± 1.13 | 10.24 ± 0.29 | 10.51 ± 0.65 | 3.87 ± 0.23 | 10.26 ± 1.10 | 14.63 ± 1.63 |
| 7 | 200.0 | 277 | 63.5 | 20.3 | 3.1 | 0.29 | 159.25 ± 7.09 | 59.23 ± 1.21 | 27.78 ± 1.67 | 21.12 ± 2.13 | 10.31 ± 0.48 | 10.52 ± 0.67 | 4.99 ± 0.69 | 10.77 ± 1.04 | 17.20 ± 1.82 |
| 8 | 300.0 | 60 | 67.5 | 20.5 | 3.3 | 0.31 | 148.98 ± 6.88 | 51.88 ± 2.22 | 25.76 ± 2.53 | 19.45 ± 1.76 | 9.76 ± 0.93 | 7.30 ± 1.21 | 2.65 ± 0.35 | 10.01 ± 2.03 | 12.16 ± 0.77 |
| 9 | 300.0 | 240 | 58.0 | 18.5 | 3.2 | 0.31 | 169.01 ± 4.43 | 60.16 ± 0.91 | 31.42 ± 2.34 | 24.65 ± 1.34 | 16.06 ± 0.87 | 18.51 ± 0.67 | 9.63 ± 0.19 | 17.76 ± 0.84 | 18.82 ± 0.63 |
| 10 | 341.5 | 150 | 58.0 | 18.1 | 3.5 | 0.32 | 169.64 ± 5.56 | 59.50 ± 1.14 | 28.51 ± 1.67 | 22.88 ± 1.48 | 14.01 ± 1.01 | 14.41 ± 0.87 | 8.55 ± 0.23 | 13.95 ± 1.72 | 17.38 ± 0.88 |
| Factor | Regression Coefficient (β) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield after Drying (%) | Moisture Content (%) | Antioxidant Activities (mg GAE/g DW2) | Catechin Content (mg/g DW2) | ||||||||
| TPC | DPPH | ABTS | FRAP | C | EGCG | EGC | ECG | EC | |||
| Constant (β0) | 20.555 | 3.097 | 86.116 | 25.421 | 14.721 | 21.541 | 11.907 | 5.424 | 2.117 | −4.066 | 2.214 |
| Linear | |||||||||||
| β1 | −0.45 | 0.0095 | 0.172 | 0.077 | 0.00488 | 0.00261 | −0.030 | −0.0062 | −0.019 | −0.0034 | 0.032 |
| β2 | −0.78 | −0.0039 | 0.048 | 0.057 | −0.0100 | −0.014 | −0.020 | 0.0069 | −0.0039 | 0.059 | 0.049 |
| Quadratic | |||||||||||
| β11 | −0.58 | - | - | - | - | - | 0.000060 | −0.00002 | 0.000041 | −0.000015 | −0.00007 |
| β22 | 0.12 | - | - | - | - | - | −0.00001 | −0.00013 | −0.00005 | −0.00130 | −0.00009 |
| Interaction | |||||||||||
| β12 | −0.43 | ns | 0.00043 | ns | 0.00016 | 0.00018 | 0.00017 | 0.00290 | 0.00018 | 0.0000654 | 0.00013 |
| Adj−R2 | 0.9595 | 0.690 | 0.7113 | 0.6952 | 0.7873 | 0.8053 | 0.9130 | 0.9591 | 0.9933 | 0.9422 | 0.9752 |
| F | 18.96 | 5.05 | 4.93 | 6.65 | 7.40 | 8.27 | 8.39 | 18.76 | 42.19 | 13.03 | 31.5 |
| p-value | 0.0069 | 0.0439 | 0.0466 | 0.0241 | 0.0193 | 0.0149 | 0.0303 | 0.0070 | 0.0233 | 0.0138 | 0.0026 |
| Lack of fit | 0.4162 | 0.5208 | 0.3309 | 0.1293 | 0.0724 | 0.0114 | 0.0551 | 0.0214 | 0.0822 | 0.0709 | 0.5288 |
| TR | Temperature (°C) | Time (s) | Sensory Scores | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tea Leaves-Appearance | Tea Leaves-Color | Appearance | Color | Overall Odor (ns) | Green Tea Odor (ns) | Overall Flavor | Green Tea Flavor | Overall Taste | Bitter Taste | Sweet Taste | Astringent Taste | Overall Liking | |||
| 1 | 58.5 | 150 | 5.2 ± 0.5d | 5.1 ± 1.4cd | 7.0 ± 0.8 a | 6.5 ± 0.7 a | 6.9 ± 0.6 | 6,7 ± 0.9 | 5.8 ± 1.3 c | 5.1 ± 0.8 | 4.8 ± 1.0 c | 4.8 ± 1.2 d | 4.7 ± 1.3 c | 4.8 ± 1.4 d | 4.8 ± 1.4 d |
| 2 | 100.0 | 60 | 5.1 ± 1.5 de | 4.7 ± 1.6 d | 5.7 ± 1.3 b | 5.1 ± 1.2 b | 6.8 ± 0.6 | 6.4 ± 1.3 | 6.0 ± 1.0 c | 6.1 ± 1.3 bcd | 6.1 ± 1.0 b | 6.0 ± 1.1 bcd | 5.9 ± 0.8 bc | 6.0 ± 1.1 abcd | 6.2 ± 1.2 bc |
| 3 | 100.0 | 240 | 7.0 ± 05 ab | 7.0 ± 0.8 a | 7.1 ± 1.0 a | 6.8 ± 1.2 a | 6.6 ± 0.9 | 7.4 ± 0.9 | 6.5 ± 0.9 abc | 7.4 ± 0.5 cd | 6.0 ± 1.0 b | 6.4 ± 0.9 abc | 6.1 ± 1.1 ab | 6.1 ± 1.0 abcd | 6.1 ± 1.1 bcd |
| 4 | 200.0 | 22 | 5.4 ± 0.9 cd | 5.4 ± 1.1 bcd | 6.7 ± 1.3 ab | 6.7 ± 1.1 a | 6.9 ± 0.8 | 6.7 ± 1.2 | 6.7 ± 0.7 abc | 6.0 ± 1.1 bcd | 6.1 ± 1.2 b | 5.4 ± 1.3 cd | 5.4 ± 1.1 bc | 5.4 ± 1.4 cd | 5.7 ± 1.3 cd |
| 5 | 200.0 | 150 | 7.4 ± 0.7 a | 7.0 ± 0.8 a | 7.8 ± 1.0 a | 7.7 ± 1.1 a | 7.1 ± 1.2 | 7.0 ± 1.4 | 6.1 ± 0.1 bc | 6.0 ± 1.0 bcd | 6.0 ± 0.5 b | 5.6 ± 1.6 cd | 5.6 ± 1.1 bc | 5.8 ± 1.5 bcd | 6.1 ± 1.2 bcd |
| 6 | 200.0 | 150 | 6.3 ± 04 bc | 6.4 ± 0.5 ab | 7.6 ± 0.5 a | 7.1 ± 0.6 a | 6.7 ± 0.9 | 6.8 ± 1.2 | 6.7 ± 0.7 abc | 6.5 ± 0.5 abc | 6.1 ± 1.2 b | 6.4 ± 1.1 abc | 6.4 ± 0.5 ab | 6.4 ± 0.7 abc | 6.5 ± 0.7 bc |
| 7 | 200.0 | 277 | 6.6 ± 0.5 ab | 6.1 ± 1.4 abc | 7.6 ± 0.7 a | 7.4 ± 0.7 a | 7.7 ± 1.1 | 7.4 ± 1.1 | 7.1 ± 0.6 ab | 7.4 ± 0.5 a | 7.4 ± 0.7 a | 6.6 ± 0.5 abc | 6.4 ± 1.2 ab | 6.7 ± 1.2 abc | 7.1 ± 0.8 ab |
| 8 | 300.0 | 60 | 7.3 ± 0.7 ab | 6.8 ± 0.8 a | 7.1 ± 0.8 a | 7.1 ± 1.2 a | 6.4 ± 0.7 | 6.0 ± 1.1 | 6.0 ± 0.8 c | 5.8 ± 1.2 cd | 5.7 ± 0.7 bc | 6.0 ± 0.8 bcd | 5.7 ± 1.1 bc | 6.0 ± 0.8 abcd | 6.0 ± 0.8 bcd |
| 9 | 300.0 | 240 | 7.4 ± 0.8 a | 7.1 ± 0.7 a | 7.7 ± 0.7 a | 7.7 ± 0.7 a | 7.4 ± 1.1 | 7.2 ± 1.1 | 7.2 ± 0.4 a | 7.0 ± 0.8 ab | 7.5 ± 0.5 a | 7.4 ± 0.5 a | 7.4 ± 1.2 a | 7.4 ± 1.5 a | 8.0 ± 0.8 a |
| 10 | 341.5 | 150 | 7.4 ± 1.2 a | 7.1 ± 0.6 a | 7.3 ± 0.9 a | 7.0 ± 1.2 a | 6.8 ± 1.5 | 6.7 ± 1.2 | 6.9 ± 0.8 abc | 6.3 ± 1.1 a | 7.5 ± 0.9 a | 7.2 ± 1.3 ab | 6.7 ± 1.3 ab | 7.0 ± 1.2 ab | 7.2 ± 0.9 ab |
| Extraction Technique | |||
|---|---|---|---|
| MAE | UAE | TDE | |
| Extraction yield (%, DW) | 8.46 ± 1.43 a | 4.02 ± 0.88 b | 3.25 ± 0.65 c |
| Moisture content (%) | 12.21 ± 2.74 b | 13.09 ± 1.23 a | 11.55 ± 1.74 c |
| TPC (mg GAE/g DW) | 462.87 ± 8.23 a | 395.13 ± 10.64 b | 378.63 ± 7.71 c |
| DPPH (mg GAE/g DW) | 165.02 ± 13.48 a | 130.71 ± 14.11 b | 127.05 ± 10.55 b |
| C (mg/g DW) | 72.53 ± 6.37 a | 48.08 ± 6.24 b | 41.86 ± 2.61 c |
| EGCG (mg/g DW) | 76.74 ± 7.76 a | 52.99 ± 4.94 b | 49.77 ± 16.62 b |
| EGC (mg/g DW) | 52.25 ± 4.50 a | 40.19 ± 8.73 b | 38.56 ± 6.91 b |
| ECG (mg/g DW) | 61.71 ± 4.91 a | 43.63 ± 1.92 b | 46.63 ± 4.41 b |
| EC (mg/g DW) | 69.6 ± 4.21 a | 44.82 ± 6.73 c | 51.46 ± 4.80 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, K.; Srichairatanakool, S.; Chewonarin, T.; Brennan, C.S.; Brennan, M.A.; Klangpetch, W.; Utama-ang, N. Manipulation of the Phenolic Quality of Assam Green Tea through Thermal Regulation and Utilization of Microwave and Ultrasonic Extraction Techniques. Horticulturae 2022, 8, 338. https://doi.org/10.3390/horticulturae8040338
Singh K, Srichairatanakool S, Chewonarin T, Brennan CS, Brennan MA, Klangpetch W, Utama-ang N. Manipulation of the Phenolic Quality of Assam Green Tea through Thermal Regulation and Utilization of Microwave and Ultrasonic Extraction Techniques. Horticulturae. 2022; 8(4):338. https://doi.org/10.3390/horticulturae8040338
Chicago/Turabian StyleSingh, Kanjana, Somdet Srichairatanakool, Teera Chewonarin, Charles Stephen Brennan, Margaret Anne Brennan, Wannaporn Klangpetch, and Niramon Utama-ang. 2022. "Manipulation of the Phenolic Quality of Assam Green Tea through Thermal Regulation and Utilization of Microwave and Ultrasonic Extraction Techniques" Horticulturae 8, no. 4: 338. https://doi.org/10.3390/horticulturae8040338