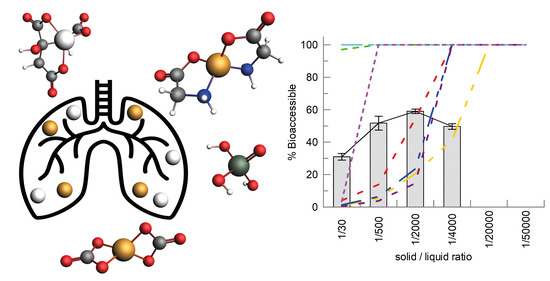
New Insights into Health Risk Assessments for Inhalational Exposure to Metal(loid)s: The Application of Aqueous Chemistry Modelling in Understanding Bioaccessibility from Airborne Particulate Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Determination of Inhalation Bioaccessibility of As, Cu, Mn, Pb and Zn from NIST 2710a
2.2. Aqueous Chemistry Simulation
2.2.1. Aqueous Chemistry Simulation Using GWB 14
2.2.2. Aqueous Chemistry Simulation Using OLI Studio 9.5
2.3. Calculation of Modelled Bioccessibilty
2.4. Evaluation of Model Performance
3. Results and Discussion
3.1. Bioaccessibility of As, Cu, Mn, Pb and Zn from NIST 2710a in Simulated Lung Fluid
3.2. Comparison of Modelled and Experimentally Determined Bioaccessibilities
3.2.1. Comparison of Modelled and Predicted Bioaccessibilities for NIST 2710a
3.2.2. Comparison of Modelled and Predicted Bioaccessibilities for NIST 1648a and BCR 038
3.3. Effect of pH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Ouyang, W.; Shu, Y.; Tian, Y.; Feng, Y.; Zhang, T.; Chen, W. Incorporating bioaccessibility into health risk assessment of heavy metals in particulate matter originated from different sources of atmospheric pollution. Environ. Pollut. 2019, 254, 113113. [Google Scholar] [CrossRef] [PubMed]
- Entwistle, J.A.; Hursthouse, A.S.; Reis, P.A.M.; Stewart, A.G. Metalliferous mine dust: Human health impacts and the potential determinants of disease in mining communities. Curr. Pollut. Rep. 2019, 5, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Hettiarachchi, E.; Paul, S.; Cadol, D.; Frey, B.; Rubasinghege, G. Mineralogy controlled dissolution of uranium from airborne dust in simulated lung fluids (slfs) and possible health implications. Environ. Sci. Tech. Lett. 2018, 6, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.R.; Elom, N.I.; Entwistle, J.A. Use of simulated epithelial lung fluid in assessing the human health risk of pb in urban street dust. Sci. Total Environ. 2017, 579, 387–395. [Google Scholar] [CrossRef]
- Davies, N.M.; Feddah, M.R. A novel method for assessing dissolution of aerosol inhaler products. Int. J. Pharm. 2003, 255, 175–187. [Google Scholar] [CrossRef]
- Lay, J.C.; Bennett, W.D.; Kim, C.S.; Devlin, R.B.; Bromberg, P.A. Retention and intracellular distribution of instilled iron oxide particles in human alveolar macrophages. Am. J. Resp. Cell Mol. 1998, 18, 687–695. [Google Scholar] [CrossRef]
- Ren, H.; Yu, Y.; An, T. Bioaccessibilities of metal (loid) s and organic contaminants in particulates measured in simulated human lung fluids: A critical review. Environ. Pollut. 2020, 265, 115070. [Google Scholar] [CrossRef]
- Caboche, J.; Perdrix, E.; Malet, B.; Alleman, L.Y. Development of an in vitro method to estimate lung bioaccessibility of metals from atmospheric particles. J. Environ. Monit. 2011, 13, 621–630. [Google Scholar]
- Kelly, F.J.; Fussell, J.C. Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radical Biol. Med. 2017, 110, 345–367. [Google Scholar] [CrossRef] [Green Version]
- Twining, J.; McGlinn, P.; Loi, E.; Smith, K.; Gieré, R. Risk ranking of bioaccessible metals from fly ash dissolved in simulated lung and gut fluids. Environ. Sci. Technol. 2005, 39, 7749–7756. [Google Scholar] [CrossRef]
- Tsuda, A.; Henry, F.S.; Butler, J.P. Particle transport and deposition: Basic physics of particle kinetics. Compr. Physiol. 2011, 3, 1437–1471. [Google Scholar]
- Boisa, N.; Elom, N.; Dean, J.R.; Deary, M.E.; Bird, G.; Entwistle, J.A. Development and application of an inhalation bioaccessibility method (ibm) for lead in the pm10 size fraction of soil. Environ. Int. 2014, 70, 132–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastury, F.; Smith, E.; Juhasz, A.L. A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal (loid) s from ambient particulate matter or dust. Sci. Total Environ. 2017, 574, 1054–1074. [Google Scholar] [CrossRef] [PubMed]
- Pelfrêne, A.; Cave, M.R.; Wragg, J.; Douay, F. In vitro investigations of human bioaccessibility from reference materials using simulated lung fluids. Int. J. Environ. Res. 2017, 14, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meza-Figueroa, D.; Barboza-Flores, M.; Romero, F.M.; Acosta-Elias, M.; Hernández-Mendiola, E.; Maldonado-Escalante, F.; Pérez-Segura, E.; González-Grijalva, B.; Meza-Montenegro, M.; García-Rico, L. Metal bioaccessibility, particle size distribution and polydispersity of playground dust in synthetic lysosomal fluids. Sci. Total Environ. 2020, 713, 136481. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.N.; Root, R.A.; Lantz, R.C.; Sáez, A.E.; Chorover, J. Oxidative weathering decreases bioaccessibility of toxic metal (loid) s in pm10 emissions from sulfide mine tailings. GeoHealth 2018, 2, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Helser, J.; Cappuyns, V. Trace elements leaching from pbzn mine waste (plombières, belgium) and environmental implications. J. Geochem. Explor. 2020, 220, 106659. [Google Scholar] [CrossRef]
- Palumbo-Roe, B.; Wragg, J.; Cave, M.R.; Wagner, D. Effect of weathering product assemblages on pb bioaccessibility in mine waste: Implications for risk management. Environ. Sci. Pollut. Res. 2013, 20, 7699–7710. [Google Scholar] [CrossRef] [Green Version]
- Ettler, V.; Šebek, O.; Grygar, T.; Klementová, M.; Bezdička, P.; Slavíková, H. Controls on metal leaching from secondary pb smelter air-pollution-control residues. Environ. Sci. Technol. 2008, 42, 7878–7884. [Google Scholar] [CrossRef]
- Kastury, F.; Karna, R.R.; Scheckel, K.G.; Juhasz, A.L. Correlation between lead speciation and inhalation bioaccessibility using two different simulated lung fluids. Environ. Pollut. 2020, 23, 114609. [Google Scholar] [CrossRef]
- Wragg, J.; Klinck, B. The bioaccessibility of lead from welsh mine waste using a respiratory uptake test. J. Environ. Sci. Health A 2007, 42, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V.; Johan, Z.; Baronnet, A.; Jankovský, F.; Gilles, C.; Mihaljevič, M.; Šebek, O.; Strnad, L.; Bezdička, P. Mineralogy of air-pollution-control residues from a secondary lead smelter: Environmental implications. Environ. Sci. Technol. 2005, 39, 9309–9316. [Google Scholar] [CrossRef] [PubMed]
- Vítková, M.; Ettler, V.; Šebek, O.; Mihaljevič, M.; Grygar, T.; Rohovec, J. The ph-dependent leaching of inorganic contaminants from secondary lead smelter fly ash. J. Haz. Mat. 2009, 167, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, J.; Maozhe, C. Minteq modeling for evaluating the leaching behavior of heavy metals in mswi fly ash. J. Environ. Sci. 2008, 20, 1398–1402. [Google Scholar] [CrossRef]
- McGivney, E.; Gustafsson, J.P.; Belyazid, S.; Zetterberg, T.; Löfgren, S. Assessing the impact of acid rain and forest harvest intensity with the hd-minteq model–soil chemistry of three swedish conifer sites from 1880 to 2080. Soil 2019, 5, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Parkhurst, D.L.; Appelo, C. Description of Input and Examples for Phreeqc Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Reston, VA, USA, 2013.
- Peterson, S.; Hostetler, C.; Deutsch, W.; Cowan, C. Minteq User’s Manual; Pacific Northwest Lab.: Richland, WA, USA, 1987. [Google Scholar]
- Ball, J.W.; Nordstrom, D.K. Wateq4f-User’s Manual with Revised Thermodynamic Data Base and Test. Cases for Calculating Speciation of Major, Trace and Redox Elements in Natural Waters; U.S. Geological Survey: Reston, VA, USA, 1991.
- Bethke, C.M.; Farrell, B.; Sharifi, M. The Geochemist’s Workbench Release 14, Gwb Essentials Guide; Aqueous Solutions LLC: Champaign, IL, USA, 2020. [Google Scholar]
- OLI Systems. A Guide to Using Oli Studio Version 9.5; OLI Systems Inc.: Ceder Knolls, NJ, USA, 2016. [Google Scholar]
- Davies, L.A.; Dargue, A.; Dean, J.R.; Deary, M.E. Use of 24 khz ultrasound to improve sulfate precipitation from wastewater. Ultrason. Sonochem. 2015, 23, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Govan, P. Manipulating Aqueous Chemistry Environments in Extractive Metallurgy. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2010. [Google Scholar]
- Anawati, J.; Azimi, G. Recovery and separation of phosphorus as dicalcium phosphate dihydrate for fertilizer and livestock feed additive production from a low-grade phosphate ore. RSC Adv. 2020, 10, 38640–38653. [Google Scholar] [CrossRef]
- Okorie, A.; Entwistle, J.; Dean, J.R. The optimization of microwave digestion procedures and application to an evaluation of potentially toxic element contamination on a former industrial site. Talanta 2010, 82, 1421–1425. [Google Scholar] [CrossRef]
- Bethke, C.M. Geochemical and Biogeochemical Reaction Modeling; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Deary, M.E.; Uapipatanakul, S. Evaluation of the performance of adms in predicting the dispersion of sulfur dioxide from a complex source in Southeast Asia: Implications for health impact assessments. Air Qual. Atmos. Health 2014, 7, 381–399. [Google Scholar] [CrossRef]
- Patryla, L.; Galeriua, D. Statistical Performances Measures—Models Comparison; CEA: Paris, France, 2011. [Google Scholar]
- Li, S.-W.; Li, H.-B.; Luo, J.; Li, H.-M.; Qian, X.; Liu, M.-M.; Bi, J.; Cui, X.-Y.; Ma, L.Q. Influence of pollution control on lead inhalation bioaccessibility in pm2. 5: A case study of 2014 Youth Olympic Games in Nanjing. Environ. Int. 2016, 94, 69–75. [Google Scholar] [CrossRef]
- Martin, R.; Dowling, K.; Nankervis, S.; Pearce, D.; Florentine, S.; McKnight, S. In vitro assessment of arsenic mobility in historical mine waste dust using simulated lung fluid. Environ Geochem. Health 2018, 40, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Ebdon, L.; Fitzpatrick, S.; Foulkes, M.E. The speciation of arsenic compounds. Chem Anal. Warsaw 2002, 47, 179–188. [Google Scholar]
- Vodyanitskii, Y.N. Determination of the oxidation states of metals and metalloids: An analytical review. Eurasian Soil Sci. 2013, 46, 1139–1149. [Google Scholar] [CrossRef]



| SRM Certified Values/mg kg−1 | |||
|---|---|---|---|
| Element | NIST 2710a (Montana Soil, This Work) | NIST 1648a (Urban Atmospheric PM [8]) | BCR 038 (Fly Ash [8]) |
| As | 1540 ± 100 (1450) | 115.5 ± 3.9 | 48 ± 2.3 |
| Cu | 3420 ± 50 (3430) | 610 ± 70 | 176 ± 9 |
| Mn | 2140 ± 60 (1740) | 790 ± 44 | 479 ± 16 |
| Pb | 5520 ± 30 (5360) | 6550 ± 330 | 262 ± 11 |
| Zn | 4180 ± 150 (4100) | 4800 ± 270 | 581 ± 29 |
| Bioaccessible Concentrations/mg kg−1 (with % Bioaccessibility in Parentheses) | |||||
|---|---|---|---|---|---|
| S/L Ratio/g mL−1 | As | Cu | Pb | Mn | Zn |
| Without DPPC | |||||
| 1/4000 | 580 ± 49.8 | 1700 ± 28.9 | 531 ± 25.4 | 648 ± 49.0 | 585 ± 32.9 |
| (37.7 ± 3.2) | (49.7 ± 0.8) | (9.6 ± 0.5) | (30.3 ± 2.3) | (14 ± 0.8) | |
| 1/2000 | 495 ± 11.2 | 2020 ± 27.2 | 228 ± 20.5 | 812 ± 140 | 511 ± 14.8 |
| (32.1 ± 0.7) | (59.1 ± 0.8) | (4.1 ± 0.4) | (37.9 ± 6.5) | (12.2 ± 0.4) | |
| 1/500 | 328 ± 17.1 | 1770 ± 75.8 | 114 ± 11.8 | 602 ± 29.9 | 253 ± 24.6 |
| (21.3 ± 1.1) | (51.8 ± 2.2) | (2.1 ± 0.2) | (28.1 ± 1.4) | (6.1 ± 0.6) | |
| 1/30 | 20.4 ± 0.61 | 1060 ± 21.7 | 10.1 ± 0.43 | 367 ± 6.6 | 78.5 ± 2.43 |
| (1.3 ± 0.0) | (31 ± 0.6) | (0.2 ± 0.0) | (17.1 ± 0.3) | (1.9 ± 0.1) | |
| With DPPC | |||||
| 1/4000 | 597 ± 46.7 | 1950 ± 114 | 324 ± 19.7 | 802 ± 53.3 | 635 ± 23.5 |
| (38.8 ± 3) | (57 ± 3.3) | (5.9 ± 0.4) | (37.5 ± 2.5) | (15.2 ± 0.6) | |
| 1/2000 | 484 ± 21.8 | 1920 ± 81.9 | 217 ± 5.36 | 748 ± 74.9 | 525 ± 38.3 |
| (31.4 ± 1.4) | (56.1 ± 2.4) | (3.9 ± 0.1) | (35 ± 3.5) | (12.6 ± 0.9) | |
| 1/500 | 336 ± 6.19 | 1760 ± 69.2 | 57.3 ± 3.79 | 602 ± 24.6 | 254 ± 23.3 |
| (21.8 ± 0.4) | (51.5 ± 2) | (1.0 ± 0.1) | (28.1 ± 1.1) | (6.1 ± 0.6) | |
| 1/30 | 17.9 ± 1.3 | 1090 ± 14.4 | 9.58 ± 0.26 | 383 ± 7.70 | 82.8 ± 3.53 |
| (1.2 ± 0.1) | (31.9 ± 0.4) | (0.2 ± 0.0) | (17.9 ± 0.4) | (2.0 ± 0.1) | |
| PTE | This Work | Pelfrêne et al. [14] (Gamble) | Pelfrêne et al. [14] (Modified Gamble—Containing DPPC) | Meza-Figueroa et al. [15] |
|---|---|---|---|---|
| As | 32.1 | - | - | |
| Cu | 59.1 | 52.1 | 47.4 | 39 |
| Pb | 4.1 | 3.7 | 3.8 | 0.1 |
| Mn | 37.9 | 37.3 | 36.6 | 22.6 |
| Zn | 12.2 | 18.6 | 18.6 | 0.6 |
| Database | Phase | As | Cu | Pb | Mn | Zn |
|---|---|---|---|---|---|---|
| GWB default (pH 6.40) | Aq | HAsO42− H2AsO4− | Cu2+ CuOH+ CuCl+ | PbCO3 | Mn2+ MnHCO3+ MnSO4 | Zn2+ ZnCl+ ZnCl2 ZnSO4 |
| Solid | Cu2CO3(OH)2 | PbCO3 PbHCO3+ | ZnCr2O4 | |||
| MINTEQ (pH 7.057) | Aq | HAsO42− H2AsO4− H3AsO3 H2AsO3− | CuCO3(aq) Cu(CO3)22− CuHCO3+ Cu2+ | PbCO3 PbHCO3+ | Mn2+ MnCO3(aq) MnHCO3+ | Zn2+ ZnHCO3+ ZnCO3(aq) ZnCl+ ZnCl2(aq) |
| Solid | Cu2CO3(OH)2 | Pb3(PO4)2 | MnHPO4 | Zn(CO3) | ||
| MINTEQ + citr. and gly. (pH 7.11) | Aq | HAsO42− H2AsO4− H3AsO3 H2AsO3− | Cu-(glycine)2 Cu-glycine+ Cu-citrate− CuCO3(aq) | PbCO3 PbHCO3+ | Mn-citrate− Mn2+ MnCO3(aq) MnHCO3+ | Zn-citrate− Zn-(citrate)23− Zn-glycine+ Zn2+ ZnHCO3+ |
| Solid | Cu2CO3(OH)2 | Pb3(PO4)2 | MnHPO4 | |||
| WATEQ4F (pH 7.05) | Aq | HAsO42− H2AsO4− | CuCO3(aq) CuHCO3+ Cu(CO3)22− Cu(OH)2(aq) Cu2+ | No significant aqueous species | MnHCO3+ Mn2+ | ZnHCO3+ Zn(CO3)2− ZnCO3 Zn2+ ZnCl+ |
| Solid | Cu2CO3(OH)2 | Pb5(PO4)3Cl | MnHPO4 | ZnSiO3 | ||
| PHREEQC (pH 6.96) | Aq | - | Cu2+ Cu(OH)2 CuOH+ CuSO4 | PbCO3 PbHCO3+ Pb(CO3)22− PbCl+ Pb2+ | MnHCO3+ Mn2+ MnCO3 MnCl+ MnHCO3+ | ZnHCO3+ Zn(CO3)22− ZnCO3 Zn2+ ZnCl+ |
| Solid | - | Pb(OH)2 | MnCO3 | |||
| OLI + citr. and gly. (pH 7.00) | Aq | HAsO42− | Cu-(glycine)2 Cu-glycine+ Cu-citrate− CuCO3 CuNH32+ | PbCO3 PbOH+ Pb(CO3)22− PbCl+ Pb2+ | Mn-citrate− Mn2+ MnCl+ MnSO4 Mn-glycine+ | Zn-citrate− Zn2+ Zn-glycine+ ZnHCO3+ ZnCl+ |
| Solid | Cu2(OH)2CO3 | PbCO3 | MnCO3 | |||
| OLI (pH 7.00) | Aq | HAsO42− | CuCO3 CuNH32+ Cu(CO3)22− Cu2+ CuCl+ | PbCO3 PbOH+ Pb(CO3)22− PbCl+ Pb2+ | Mn2+ MnCl+ MnSO4 | Zn2+ ZnHCO3+ ZnCl+ ZnOH+ ZnCl2 |
| Solid | Cu3(PO4)2·2H2O | PbCO3 | MnCO3 |
| Statistical Measure and Metal | GWB Thermo | MINTEQ | MINTEQ + gly. and citr. | PHREEQC | WAT4QF | OLI + gly. and citr. | OLI |
|---|---|---|---|---|---|---|---|
| FBmean | |||||||
| As | −1.25 | −1.25 | −1.25 | - | −1.25 | −1.25 | −1.25 |
| Cu | 0.11 | 0.95 | −0.70 | −0.70 | 0.37 | −0.45 | 0.47 |
| Pb | −1.85 | 1.62 | 1.68 | −1.76 | 1.67 | −0.71 | −0.44 |
| Mn | −1.05 | 1.69 | 1.64 | −0.47 | 1.92 | 0.93 | 1.19 |
| Zn | −1.68 | −1.48 | −1.62 | −1.69 | −0.25 | −1.69 | −1.69 |
| NMSE | |||||||
| As | 2.64 | 2.64 | 2.64 | - | 2.64 | 2.64 | 2.64 |
| Cu | 0.52 | 1.29 | 0.57 | 0.59 | 1.00 | 0.45 | 1.28 |
| Pb | 23.07 | 7.39 | 9.80 | 12.98 | 8.96 | 0.58 | 0.18 |
| Mn | 1.49 | 9.22 | 7.75 | 0.91 | 43.19 | 1.62 | 3.40 |
| Zn | 9.55 | 5.26 | 7.45 | 9.83 | 0.22 | 9.83 | 9.83 |
| Statistical Measure and Metal | GWB Thermo | MINTEQ | MINTEQ + gly. and citr. | PHREEQC | WAT4QF | OLI + gly. and citr. | OLI |
|---|---|---|---|---|---|---|---|
| FBmean | |||||||
| Pb | −1.68 | 0.78 | 0.98 | −1.58 | 0.84 | −1.42 | −1.42 |
| Pb (all) | −1.72 | 0.87 | 1.06 | −1.62 | 0.93 | −1.38 | −1.37 |
| Zn | −0.58 | −0.18 | −0.50 | −0.70 | 0.75 | −0.67 | −0.62 |
| Zn (all) | −0.81 | −0.41 | −0.72 | −0.90 | 0.66 | −0.88 | −0.84 |
| NMSE | |||||||
| Pb | 9.72 | 4.64 | 5.22 | 8.75 | 5.01 | 10.81 | 10.84 |
| Pb (all) | 11.50 | 4.75 | 5.40 | 9.40 | 5.15 | 10.40 | 10.45 |
| Zn | 0.44 | 1.20 | 0.79 | 0.72 | 5.78 | 0.63 | 0.65 |
| Zn (all) | 0.99 | 1.54 | 1.24 | 1.25 | 5.41 | 1.16 | 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deary, M.E.; Amaibi, P.M.; Dean, J.R.; Entwistle, J.A. New Insights into Health Risk Assessments for Inhalational Exposure to Metal(loid)s: The Application of Aqueous Chemistry Modelling in Understanding Bioaccessibility from Airborne Particulate Matter. Geosciences 2021, 11, 47. https://doi.org/10.3390/geosciences11020047
Deary ME, Amaibi PM, Dean JR, Entwistle JA. New Insights into Health Risk Assessments for Inhalational Exposure to Metal(loid)s: The Application of Aqueous Chemistry Modelling in Understanding Bioaccessibility from Airborne Particulate Matter. Geosciences. 2021; 11(2):47. https://doi.org/10.3390/geosciences11020047
Chicago/Turabian StyleDeary, Michael E., Patrick M. Amaibi, John R. Dean, and Jane A. Entwistle. 2021. "New Insights into Health Risk Assessments for Inhalational Exposure to Metal(loid)s: The Application of Aqueous Chemistry Modelling in Understanding Bioaccessibility from Airborne Particulate Matter" Geosciences 11, no. 2: 47. https://doi.org/10.3390/geosciences11020047