Ethanolic Extracts of Datura innoxia Have Promising Acaricidal Activity against Rhipicephalus microplus as It Blocks the Glutathione S-Transferase Activity of the Target Tick
Abstract
:1. Introduction
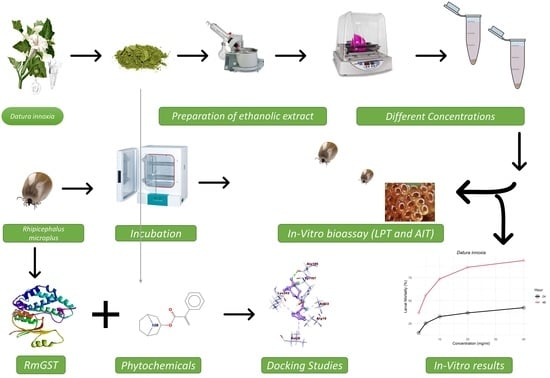
2. Materials and Methods
2.1. Preparation of Plant Extracts
2.2. Collection of R. microplus Ticks
2.3. Adult Immersion Test (AIT)
2.4. Larval Packet Test (LPT)
2.5. Glutathione S-Transferases Protein Sequence of R. microplus
2.6. Modeling the 3D Structure of RmGST
2.7. Modeled Structure Validation
2.8. Determining Binding Sites
2.9. Ligands, Protein Preparation for Molecular Docking
2.10. Statistical Analysis
3. Results
3.1. Adult Immersion Test (AIT)
3.2. Larval Packet Test (LPT)
3.3. Analysis of Protein Models and Validation
3.4. Active Site Prediction
3.5. Docking Studies
3.6. Molecular Dynamics Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patoliya, P.; Raval, K.; Upadhyaya, V.; Dewry, R.K.; Maiti, S.; Mondal, G.; Mohanty, T.K.; Bhakat, M. Tick infestation and its herbal treatment approach in India: A review. Pharma Innov. J. 2022, SP-11, 1323–1339. [Google Scholar]
- Estrada-Peña, A.; García, Z.; Sánchez, H.F. The Distribution and Ecological Preferences of Boophilus microplus (Acari: Ixodidae) in Mexico. Exp. Appl. Acarol. 2006, 38, 307–316. [Google Scholar] [CrossRef]
- Grisi, L.; Leite, R.C.; Martins, J.R.; Barros, A.T.; Andreotti, R.; Cançado, P.H.; León, A.A.; Pereira, J.B.; Villela, H.S. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miraballes, C.; Riet-Correa, F. A review of the history of research and control of Rhipicephalus (Boophilus) microplus, babesiosis and anaplasmosis in Uruguay. Exp. Appl. Acarol. 2018, 75, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.Z.; Zaman, M.A.; Colwell, D.D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20. [Google Scholar] [CrossRef]
- Luns, D.A.R.; Martins, R.; Pombal, S.; Rodilla, J.M.L.; Githaka, N.W.; Vaz, I.d.S.; Logullo, C. Effect of essential oils against acaricide-susceptible and acaricide-resistant Rhipicephalus ticks. Exp. Appl. Acarol. 2021, 83, 597–608. [Google Scholar] [CrossRef]
- Alota, S.L.; Edquiban, T.R.J.; Galay, R.L.; Bernardo, J.M.G.; Sandalo, K.A.C.; Divina, B.P.; Tanaka, T. Determination of resistance status to amitraz in the cattle tick Rhipicephalus (Boophilus) microplus from Luzon, Philippines, through bioassay and molecular analysis. Exp. Appl. Acarol. 2021, 83, 399–409. [Google Scholar] [CrossRef]
- De Souza Chagas, A.C.; de Sena Oliveira, M.C.; Giglioti, R.; Santana, R.C.M.; Bizzo, H.R.; Gama, P.E.; Chaves, F.C.M. Efficacy of 11 Brazilian essential oils on lethality of the cattle tick Rhipicephalus (Boophilus) microplus. Ticks Tick-Borne Dis. 2016, 7, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Rosado-Aguilar, J.A.; Aguilar-Caballero, A.; Rodriguez-Vivas, R.I.; Borges-Argaez, R.; Garcia-Vazquez, Z.; Mendez-Gonzalez, M. Acaricidal activity of extracts from Petiveria alliacea (Phytolaccaceae) against the cattle tick, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet. Parasitol. 2010, 168, 299–303. [Google Scholar] [CrossRef]
- Adenubi, O.T.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. In vitro bioassays used in evaluating plant extracts for tick repellent and acaricidal properties: A critical review. Vet. Parasitol. 2018, 254, 160–171. [Google Scholar] [CrossRef]
- De Souza Chagas, A.C.; de Barros, L.D.; Cotinguiba, F.; Furlan, M.; Giglioti, R.; de Sena Oliveira, M.C.; Bizzo, H.R. In vitro efficacy of plant extracts and synthesized substances on Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol. Res. 2012, 110, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Juliet, S.; Ravindran, R.; Ramankutty, S.A.; Gopalan, A.K.K.; Nair, S.N.; Kavillimakkil, A.K.; Bandyopadhyay, A.; Rawat, A.K.S.; Ghosh, S. Jatropha curcas (Linn) leaf extract–a possible alternative for population control of Rhipicephalus (Boophilus) annulatus. Asian Pac. J. Trop. Dis. 2012, 2, 225–229. [Google Scholar] [CrossRef]
- Kamaraj, C.; Rahuman, A.A.; Bagavan, A.; Elango, G.; Rajakumar, G.; Zahir, A.A.; Marimuthu, S.; Santhoshkumar, T.; Jayaseelan, C. Evaluation of medicinal plant extracts against blood-sucking parasites. Parasitol. Res. 2010, 106, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Sunil, A.R.; Amithamol, K.K.; Juliet, S.; Nair, S.N.; Ajithkumar, K.G.; Soorya, V.C.; Divya, T.M.; Jyothymol, G.; Ghosh, S.; Ravindran, R. Acaricidal effect of Cassia fistula Linn. leaf ethanolic extract against Rhipicephlaus (Boophilus) annulatus. Trop. Biomed. 2013, 30, 231–237. [Google Scholar]
- Ghosh, S.; Sharma, A.K.; Kumar, S.; Tiwari, S.S.; Rastogi, S.; Srivastava, S.; Singh, M.; Kumar, R.; Paul, S.; Ray, D.D. In vitro and in vivo efficacy of Acorus calamus extract against Rhipicephalus (Boophilus) microplus. Parasitol. Res. 2011, 108, 361–370. [Google Scholar] [CrossRef]
- Ghosh, S.; Tiwari, S.S.; Srivastava, S.; Sharma, A.K.; Kumar, S.; Ray, D.D.; Rawat, A.K.S. Acaricidal properties of Ricinus communis leaf extracts against organophosphate and pyrethroids resistant Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2013, 192, 259–267. [Google Scholar] [CrossRef]
- Bagewadi, Z.K.; Muddapur, U.M.; Madiwal, S.S.; Mulla, S.I.; Khan, A. Biochemical and enzyme inhibitory attributes of methanolic leaf extract of Datura inoxia Mill. Environ. Sustain. 2019, 2, 75–87. [Google Scholar] [CrossRef]
- Neeraj, O.M.; Ayesha, K.; Balu, A.C. Rediscovering the medicinal properties of Datura sp.: A review. J. Med. Plants Res. 2013, 7, 2885–2897. [Google Scholar]
- Ramadan, M.F.; Zayed, R.; El-Shamy, H. Screening of bioactive lipids and radical scavenging potential of some solanaceae plants. Food Chem. 2007, 103, 885–890. [Google Scholar] [CrossRef]
- Vermillion, K.; Holguin, F.O.; Berhow, M.A.; Richins, R.D.; Redhouse, T.; O’Connell, M.A.; Posakony, J.; Mahajan, S.S.; Kelly, S.M.; Simon, J.A. Dinoxin b, a withanolide from Datura inoxia leaves with specific cytotoxic activities. J. Nat. Prod. 2011, 74, 267–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guneidy, R.A.; Amer, M.A.; Hakim, A.E.E.; Abdel-Shafy, S.; Allam, S.A. Effect of polyphenols extracted from Punica granatum and Acacia saligna plants on glutathione S-transferase of the cattle tick Rhipicephalus (Boophilus) annulatus (Acari: Ixodidae). J. Parasit. Dis. 2021, 45, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Sabadin, G.A.; Parizi, L.F.; Kiio, I.; Xavier, M.A.; da Silva Matos, R.; Camargo-Mathias, M.I.; Githaka, N.W.o.; Nene, V.; da Silva Vaz, I., Jr. Effect of recombinant glutathione S-transferase as vaccine antigen against Rhipicephalus appendiculatus and Rhipicephalus sanguineus infestation. Vaccine 2017, 35, 6649–6656. [Google Scholar] [CrossRef] [PubMed]
- Whiten, S.R.; Eggleston, H.; Adelman, Z.N. Ironing out the details: Exploring the role of iron and heme in blood-sucking arthropods. Front. Physiol. 2018, 8, 1134. [Google Scholar] [CrossRef] [Green Version]
- Balcı, N.; Türkan, F.; Şakiroğlu, H.; Aygün, A.; Şen, F. Purification and characterization of glutathione S-transferase from blueberry fruits (Vaccinium arctostaphylos L.) and investigated of some pesticide inhibition effects on enzyme activity. Heliyon 2019, 5, e01422. [Google Scholar] [CrossRef] [Green Version]
- Guneidy, R.A.; Shahein, Y.E.; Abouelella, A.M.K.; Zaki, E.R.; Hamed, R.R. Inhibition of the recombinant cattle tick Rhipicephalus (Boophilus) annulatus glutathione S-transferase. Ticks Tick-Borne Dis. 2014, 5, 528–536. [Google Scholar] [CrossRef]
- Hernandez, E.P.; Kusakisako, K.; Talactac, M.R.; Galay, R.L.; Hatta, T.; Matsuo, T.; Fujisaki, K.; Tsuji, N.; Tanaka, T. Characterization and expression analysis of a newly identified glutathione S-transferase of the hard tick Haemaphysalis longicornis during blood-feeding. Parasites Vectors 2018, 11, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sookrung, N.; Reamtong, O.; Poolphol, R.; Indrawattana, N.; Seesuay, W.; Saelim, N.; Tantilipikorn, P.; Bunnag, C.; Chaicumpa, W.; Tungtrongchitr, A. Glutathione S-transferase (GST) of American cockroach, Periplaneta americana: Classes, isoforms, and allergenicity. Sci. Rep. 2018, 8, 484. [Google Scholar] [CrossRef] [Green Version]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 2167-0412. [Google Scholar]
- Shyma, K.P.; Gupta, J.P.; Ghosh, S.; Patel, K.K.; Singh, V. Acaricidal effect of herbal extracts against cattle tick Rhipicephalus (Boophilus) microplus using in vitro studies. Parasitol. Res. 2014, 113, 1919–1926. [Google Scholar] [CrossRef]
- Stendel, W. The relevance of different test methods for the evaluation of tick controlling substances. J. S Afr. Vet. Assoc. 1980, 51, 147–152. [Google Scholar] [PubMed]
- Sabatini, G.A.; Kemp, D.H.; Hughes, S.; Nari, A.; Hansen, J. Tests to determine LC50 and discriminating doses for macrocyclic lactones against the cattle tick, Boophilus microplus. Vet. Parasitol. 2001, 95, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Anishchenko, I.; Park, H.; Peng, Z.; Ovchinnikov, S.; Baker, D. Improved protein structure prediction using predicted interresidue orientations. Proc. Natl. Acad. Sci. USA 2020, 117, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Agyapong, O.; Asiedu, S.O.; Kwofie, S.K.; Miller Iii, W.A.; Parry, C.S.; Sowah, R.A.; Wilson, M.D. Molecular modelling and de novo fragment-based design of potential inhibitors of beta-tubulin gene of Necator americanus from natural products. Inform. Med. Unlocked 2021, 26, 100734. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K. DS Goodsell i AJ Olson. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing, 4.1.3; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2022. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical procedures for agricultural research. R Package Version 2014, 1, 1–4. [Google Scholar]
- Shezryna, S.; Anisah, N.; Saleh, I.; Syamsa, R.A. Acaricidal activity of the essential oils from Citrus hystrix (Rutaceae) and Cymbopogon citratus (Poaceae) on the cattle tick Rhipicephalus (Boophilus) microplus larvae (Acari: Ixodidae). Trop. Biomed. 2020, 37, 433–442. [Google Scholar]
- Finney, D. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Hlina, B.L.; Birceanu, O.; Robinson, C.S.; Dhiyebi, H.; Wilkie, M.P. The relationship between thermal physiology and lampricide sensitivity in larval sea lamprey (Petromyzon marinus). J. Great Lakes Res. 2021, 47, S272–S284. [Google Scholar] [CrossRef]
- Wickham, H. Data analysis. In Ggplot2; Springer: Cham, Switzerland, 2016; pp. 189–201. [Google Scholar]
- Eisenberg, D.; Lüthy, R.; Bowie, J.U. [20] VERIFY3D: Assessment of protein models with three-dimensional profiles. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 277, pp. 396–404. [Google Scholar]
- Bowie, J.U.; Lüthy, R.; Eisenberg, D. A method to identify protein sequences that fold into a known three-dimensional structure. Science 1991, 253, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; Luscombe, N.M.; Swindells, M.B.; Thornton, J.M. Protein clefts in molecular recognition and function. Protein Sci. A Publ. Protein Soc. 1996, 5, 2438. [Google Scholar]
- Liang, J.; Woodward, C.; Edelsbrunner, H. Anatomy of protein pockets and cavities: Measurement of binding site geometry and implications for ligand design. Protein Sci. 1998, 7, 1884–1897. [Google Scholar] [CrossRef] [Green Version]
- Broni, E.; Kwofie, S.K.; Asiedu, S.O.; Miller Iii, W.A.; Wilson, M.D. A molecular modeling approach to identify potential antileishmanial compounds against the cell division cycle (Cdc)-2-related kinase 12 (crk12) receptor of leishmania donovani. Biomolecules 2021, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Kwofie, S.K.; Broni, E.; Yunus, F.U.; Nsoh, J.; Adoboe, D.; Miller Iii, W.A.; Wilson, M.D. Molecular Docking Simulation Studies Identifies Potential Natural Product Derived-Antiwolbachial Compounds as Filaricides against Onchocerciasis. Biomedicines 2021, 9, 1682. [Google Scholar] [CrossRef]
- Ghosh, S.; Tiwari, S.S.; Kumar, B.; Srivastava, S.; Sharma, A.K.; Kumar, S.; Bandyopadhyay, A.; Julliet, S.; Kumar, R.; Rawat, A.K.S. Identification of potential plant extracts for anti-tick activity against acaricide resistant cattle ticks, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Exp. Appl. Acarol. 2015, 66, 159–171. [Google Scholar] [CrossRef]
- Rosado-Aguilar, J.A.; Arjona-Cambranes, K.; Torres-Acosta, J.F.J.; Rodríguez-Vivas, R.I.; Bolio-González, M.E.; Ortega-Pacheco, A.; Alzina-López, A.; Gutiérrez-Ruiz, E.J.; Gutiérrez-Blanco, E.; Aguilar-Caballero, A.J. Plant products and secondary metabolites with acaricide activity against ticks. Vet. Parasitol. 2017, 238, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Tiwari, S.S.; Srivastava, S.; kumar, S.; Sharma, A.K.; Nagar, G.; Kumar, K.G.A.; Kumar, R.; Rawat, A.K.S. In vitro acaricidal properties of Semecarpus anacardium fruit and Datura stramonium leaf extracts against acaricide susceptible (IVRI-I line) and resistant (IVRI-V line) Rhipicephalus (Boophilus) microplus. Res. Vet. Sci. 2015, 101, 69–74. [Google Scholar] [CrossRef]
- Kumral, N.A.; Çobanoğlu, S.; Yalcin, C. Acaricidal, repellent and oviposition deterrent activities of Datura stramonium L. against adult Tetranychus urticae (Koch). J. Pest Sci. 2010, 83, 173–180. [Google Scholar] [CrossRef]
- Singla, R.K. In silico drug design & medicinal chemistry. Curr. Top. Med. Chem. 2015, 15, 971. [Google Scholar] [PubMed]
- Wang, C.; Xu, P.; Zhang, L.; Huang, J.; Zhu, K.; Luo, C. Current strategies and applications for precision drug design. Front. Pharmacol. 2018, 9, 787. [Google Scholar] [CrossRef]
- Zheng, M.; Zhao, J.; Cui, C.; Fu, Z.; Li, X.; Liu, X.; Ding, X.; Tan, X.; Li, F.; Luo, X. Computational chemical biology and drug design: Facilitating protein structure, function, and modulation studies. Med. Res. Rev. 2018, 38, 914–950. [Google Scholar] [CrossRef] [PubMed]





| Extract | Concentration (mg/mL) | % Mean ± Standard Deviation | ||
|---|---|---|---|---|
| % Larval Mortality | % Inhibition of Oviposition (IO) | |||
| 24 h | 48 h | |||
| D. innoxia | 40 | 42.33 ± 2.08 b | 93.67 ± 1.53 a | 45.37 ± 2.60 b |
| 20 | 36.67 ± 2.08 bc | 86.33 ± 6.66 ab | 32.85 ± 1.63 c | |
| 10 | 32.67 ± 3.06 c | 73.67 ± 5.13 b | 20.68 ± 2.80 d | |
| 5 | 25.33 ± 2.52 d | 55.67 ± 8.02 c | 15.73 ± 5.98 de | |
| 2.5 | 15.00 ± 1.00 e | 36.67 ± 1.53 d | 9.86 ± 4.01 ef | |
| Control Group | Trichlorfon | 58.00 ± 3.00 a | 97.67 ± 2.52 a | 83.74 ± 5.02 a |
| Distilled water | 0 f | 1.00 ± 1.00 e | 0.20 ± 1.46 f | |
| Time (h) | LC50 (mg/mL) | 95% Confidence Limits | LC90 (mg/mL) | 95% Confidence Limits | Slope ± S.E. | Intercept ± S.E. | Chi-Square (χ2) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LCL | UCL | LCL | UCL | |||||||
| 24 | 66.44 | 43.79 | 127.55 | 6310.72 | 1754.24 | 52,277.92 | 0.64 ± 0.08 | −1.18 ± 0.09 | 6.48 | 0.93 |
| 48 | 4.060 | 3.530 | 4.586 | 26.632 | 22.564 | 32.56 | 1.56 ± 0.09 | −0.95 ± 0.09 | 16.51 | 0.22 |
| Concentration (mg/mL) | LT50 (h) | 95% Confidence Limits | LT90 (h) | 95% Confidence Limits | Slope ± S. E | Intercept ± SE | Chi-Square (χ2) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LCL | UCL | LCL | UCL | |||||||
| 2.5 | 67.39 | 56.21 | 93.62 | 241.63 | 150.14 | 608.63 | 2.31 ± 0.38 | −4.22 ± 0.59 | 0.35 | 0.98 |
| 5 | 42.46 | 38.67 | 47.99 | 127.75 | 96.59 | 203.91 | 2.67 ± 0.35 | −4.36 ± 0.55 | 5.88 | 0.21 |
| 10 | 31.99 | 29.78 | 34.25 | 72.7173 | 63.12 | 89.09 | 3.59 ± 0.35 | −5.41 ± 0.55 | 3.56 | 0.47 |
| 20 | 28.28 | 26.62 | 29.88 | 52.51 | 48.17 | 58.76 | 4.77 ± 0.38 | −6.92 ± 0.58 | 7.88 | 0.09 |
| 40 | 25.94 | 24.53 | 27.26 | 43.47 | 40.57 | 47.46 | 5.71 ± 0.47 | −8.08 ± 0.65 | 1.14 | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saman, S.; Chen, C.-C.; Malak, N.; Khan, A.; Nasreen, N.; Khan, A.; Niaz, S.; Rehman, G.; Rodriguez-Vivas, R.I.; Cossío-Bayúgar, R. Ethanolic Extracts of Datura innoxia Have Promising Acaricidal Activity against Rhipicephalus microplus as It Blocks the Glutathione S-Transferase Activity of the Target Tick. Genes 2023, 14, 118. https://doi.org/10.3390/genes14010118
Saman S, Chen C-C, Malak N, Khan A, Nasreen N, Khan A, Niaz S, Rehman G, Rodriguez-Vivas RI, Cossío-Bayúgar R. Ethanolic Extracts of Datura innoxia Have Promising Acaricidal Activity against Rhipicephalus microplus as It Blocks the Glutathione S-Transferase Activity of the Target Tick. Genes. 2023; 14(1):118. https://doi.org/10.3390/genes14010118
Chicago/Turabian StyleSaman, Saman, Chien-Chin Chen, Nosheen Malak, Afshan Khan, Nasreen Nasreen, Adil Khan, Sadaf Niaz, Gauhar Rehman, Roger I. Rodriguez-Vivas, and Raquel Cossío-Bayúgar. 2023. "Ethanolic Extracts of Datura innoxia Have Promising Acaricidal Activity against Rhipicephalus microplus as It Blocks the Glutathione S-Transferase Activity of the Target Tick" Genes 14, no. 1: 118. https://doi.org/10.3390/genes14010118