Cryogel Scaffolds for Tissue-Engineering: Advances and Challenges for Effective Bone and Cartilage Regeneration
Abstract
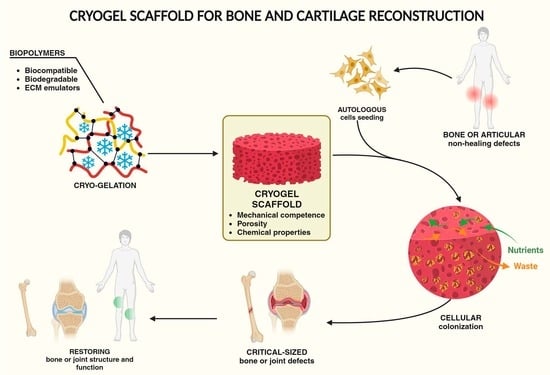
:1. Introduction
2. Bone and Cartilage Reconstruction with Cryogel Scaffolds
3. Cryogel Fabrication
- Solubilization of the matrix components, which can be represented by monomers or macromers, and the cross-linking agents;
- Cryo-gelation;
- Solvent crystals removal.
3.1. Solubilization of the Matrix Components
3.2. Freezing and Cryo-Gelation
3.3. Freezing Methods
3.4. Polymer Cross-Linking
3.5. Solvent Crystals Removal
4. Material Used for the Fabrication of Cryogels Suitable for Bone and Cartilage Reconstruction
4.1. Collagen-Based Cryogels
4.2. Gelatin-Based Cryogels
4.3. Silk Fibroin-Based Cryogels
4.4. Glycosaminoglycans (GAGs)
4.5. Other Natural Polymers
| Principal Cryogel Component | Key Properties | Blended Organic Materials | Inorganic Additives | Cross-Linking Method | Refs. |
|---|---|---|---|---|---|
| Collagen | ECM component; RGD sequences | gelatin; elastin | EDC/NHS | [51] | |
| carboxymethyl cellulose | TCP | physical cross-linking | [33] | ||
| HA | EDC/NHS | [71] | |||
| keratin | silica | EDC/NHS | [99] | ||
| HA | GA | [46] | |||
| hyaluronic acid methacrylate | GA | [32] | |||
| chitosan; fucoidan; chondroitin sulfate | physical cross-linking | [2,100] | |||
| Gelatin | ECM emulator; RGD sequences; MMPs binding sites; stiffness | chitosan | GA | [15] | |
| hyaluronic acid | EDC/NHS | [50] | |||
| DexOx | HA | Schiff base reaction | [101] | ||
| p-HEMA | APS/TEMED | [102] | |||
| DexOx | HA | Schiff base reaction | [6] | ||
| hyaluronic acid; chondroitin sulfate; chitosan | EDC/NHS | [89] | |||
| HA | EDC/NHS | [70] | |||
| GA | [74] | ||||
| GA | [75] | ||||
| HA | GA | [76] | |||
| bioglass | APS/TEMED radical cross-linking | [38] | |||
| bone morphogenic protein-2 | APS/TEMED radical cross-linking | [77] | |||
| poly(lactic-co-glycolic acid) | HA | EDC/NHS | [66] | ||
| HA | EDC/NHS | [103] | |||
| heparin | EDC/NHS | [48] | |||
| UV photocuring | [39] | ||||
| BMP-2 biomimetic peptide and VEGF | APS/TEMED | [37] | |||
| hyaluronic acid; chondroitin sulfate | EDC/NHS | [88] | |||
| carboxymethyl chitosan | EDC/NHS | [49] | |||
| APS/TEMED radical cross-linking | [9] | ||||
| alginate | Ca(II) DVS | [55] | |||
| Silk fibroin | strength; toughness; mechanical stress resistance; mechanical flexibility; | 1,4-butanediol diglycidyl ether (BDDE) or ethylene glycol diglycidyl ether (EGDE) and TEMED; | [29] | ||
| BDDE/TEMED | [60] | ||||
| N,N′-methylene-bis(acrylamide) (MBAm) or N,N,N′,N ′tetramethylenediamine (TMDA); APS/TEMED radical cross-linking | [12,95,104,105,106] | ||||
| Chondroitin sulfate | mechanical flexibility; stiffness; toughness; biological trigger for cellular differentiation | hyaluronic acid | APS/TEMED cross-linking on methacryloyl derivative | [20] | |
| gelatin; chitosan | APS/TEMED radical cross-linking | [19] | |||
| Hyaluronic acid | mechanical flexibility; stiffness; toughness; biological trigger for cellular differentiation; hydrophilicity | gelatin | EDC/NHS | [107] | |
| methoxy-PEG-acrylate or RGD sequences | APS/TEMED radical cross-linking | [90] | |||
| Chitosan | mechanical flexibility | Agarose; gelatin | GA | [92] | |
| biphasic calcium phosphate | EDC/NHS | [25] | |||
| gelatin; chondroitin sulfate; hyaluronic acid | EDC | [89] | |||
| gluconic acid | EDC/NHS | [17] | |||
| Silk fibroin | Ag/Sr doped HA | physical cross-linking | [18] | ||
| agarose | HA | physical cross-linking | [108] | ||
| gelatin | Ce/Zn doped HA | GA | [73] | ||
| gelatin | HA | GA | [44] | ||
| GA | [58] | ||||
| gelatin | EDC/NHS | [49] | |||
| Nano cellulose | micro-structuring | silica | [98] |
4.6. Synthetic Polymers
4.7. Inorganic Additives
| Principal Cryogel Component | Key Properties | Blended Organic Materials | Inorganic Additives | Cross-Linking Method | Ref. |
|---|---|---|---|---|---|
| PEG | hydrophilicity; mechanical resistance | APS/TEMED radical cross-linking of diacrylate PEG | [21] | ||
| Hyaluronic acid; chondroitin sulfate | APS/TEMED radical cross-linking of diacrylate PEG | [20] | |||
| Graphene | graphene oxide | [23] | |||
| γ-polyglutammic acid | APS/TEMED radical cross-linking of PEG-HEMA | [121] | |||
| p-HEMA or p-HPMA | hydrophilicity, thermal resistance, tribological properties for articular joint grafts | Zn/Ce substitute hydroxyapatite | APS/TEMED | [113] | |
| and APS/TEMED radical cross-linking in the presence of MBAAm (N,N′-methylene-bis-acrylamide) | [57] | ||||
| PVA | cartilage-like mechanical behavior | physical gelation | [122] | ||
| physical gelation | [123] | ||||
| HA | physical gelation | [117] | |||
| agarose | Tetraethylorthosilicate; CaCl2 | physical gelation | [56] | ||
| HA | physical gelation | [115] | |||
| HA | physical gelation | [116] |
5. Cryogels in the Biological Environment: Physical, Chemical, and Physiological Properties for Scaffold Cellularity and Body Implantability
5.1. Structural Architecture and Mechanical Properties of Cryogels for Cell Hosting
5.2. Chemical Properties of Scaffolds
5.3. Inorganic Decoration as Adjuvant for Scaffold Cellularity
5.4. Histo-Compatibility and Immune-Tolerance
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALP | Alkaline Phosphatase |
| APS | Ammonium peroxydisulfate |
| Coll | Collagen |
| CP | Calcium phosphate |
| CS | Chondroitin sulfate |
| Dex | Dextran |
| DexOx | Oxidized dextran |
| ECM | Extracellular matrix |
| FB | Freeze bath |
| GA | Glutaraldehyde |
| Gel | Gelatin |
| GelMA | Methacryloyl gelatin |
| HAc | Hyaluronic acid |
| HA | Hydroxyapatite |
| HEMA | Hydroxyethyl methacrylate |
| HPMA | Hydroxypropyl methacrylate |
| OC | Osteocalcin |
| ON | Osteonectin |
| OP | Osteopontin |
| PEG | Polyethylene glycol |
| RUNX2 | Run-related transcription factor 2 |
| SiN | Silicon nitride |
| TCP | Tricalcium phosphate |
| TEMED | N,N,N’,N’-tetrametylethylendiamine |
| UF | Unidirectional freezing |
References
- Hixon, K.R.; Eberlin, C.T.; Kadakia, P.U.; McBride-Gagyi, S.H.; Jain, E.; Sell, S.A. A Comparison of Cryogel Scaffolds to Identify an Appropriate Structure for Promoting Bone Regeneration. Biomed. Phys. Eng. Express 2016, 2, 035014. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Gelinsky, M.; Williams, D.S.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. Marine Collagen-Chitosan-Fucoidan/Chondroitin Sulfate Cryo-Biomaterials Loaded with Primary Human Cells Envisaging Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2023, 241, 124510. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Melvin, A.M.; Lin, A.Y.; Hall, A.F.; Sell, S.A. Cryogel Scaffolds from Patient-Specific 3D-Printed Molds for Personalized Tissue-Engineered Bone Regeneration in Pediatric Cleft-Craniofacial Defects. J. Biomater. Appl. 2017, 32, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Sangaj, N.; Varghese, S. Interconnected Macroporous Poly(Ethylene Glycol) Cryogels as a Cell Scaffold for Cartilage Tissue Engineering. Tissue Eng.-Part A 2010, 16, 3033–3041. [Google Scholar] [CrossRef] [PubMed]
- Inci, I.; Kirsebom, H.; Galaev, I.Y.; Mattiasson, B.; Piskin, E. Gelatin Cryogels Crosslinked with Oxidized Dextran and Containing Freshly Formed Hydroxyapatite as Potential Bone Tissue-Engineering Scaffolds. J. Tissue Eng. Regen. Med. 2013, 7, 584–588. [Google Scholar] [CrossRef]
- Hussain, Z.; Mehmood, S.; Liu, X.; Liu, Y.; Wang, G.; Pei, R. Decoding Bone-Inspired and Cell-Instructive Cues of Scaffolds for Bone Tissue Engineering. Eng. Regen. 2024, 5, 21–44. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, Y.; Xi, J.; Wang, J. Polymeric Hybrid Aerogels and Their Biomedical Applications. Soft Matter 2020, 16, 9160–9175. [Google Scholar] [CrossRef]
- Di Muzio, L.; Sergi, C.; Carriero, V.C.; Tirillò, J.; Adrover, A.; Messina, E.; Gaetani, R.; Petralito, S.; Casadei, M.A.; Paolicelli, P. Gelatin-Based Spongy and Compressive Resistant Cryogels with Shape Recovery Ability as Ideal Scaffolds to Support Cell Adhesion for Tissue Regeneration. React. Funct. Polym. 2023, 189, 105607. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.M.; Saldaña, L.; Benito-Garzón, L.; García-Carrodeguas, R.; de Aza, S.; Vilaboa, N.; Román, J.S. Feasibility of Ceramic-Polymer Composite Cryogels as Scaffolds for Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2012, 6, 421–433. [Google Scholar] [CrossRef]
- Hixon, K.R.; Eberlin, C.T.; Pendyala, M.; Alarcon de la Lastra, A.; Sell, S.A. Scaffolds for Use in Craniofacial Bone Regeneration; Methods in Molecular Biology; Humana Press Inc.: Clifton, NJ, USA, 2022; Volume 2403, p. 234. [Google Scholar]
- Bölgen, N.; Yang, Y.; Korkusuz, P.; Güzel, E.; El Haj, A.J.; Pişkin, E. 3D Ingrowth of Bovine Articular Chondrocytes in Biodegradable Cryogel Scaffolds for Cartilage Tissue Engineering. J. Tissue Eng. Regen. Med. 2011, 5, 770–779. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Hutmacher, D.; Woodfield, T.; Dalton, P.; Lewis, J. Scaffold Design and Fabrication. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2008; pp. 403–454. ISBN 978-0-12-370869-4. [Google Scholar]
- Kathuria, N.; Tripathi, A.; Kar, K.K.; Kumar, A. Synthesis and Characterization of Elastic and Macroporous Chitosan-Gelatin Cryogels for Tissue Engineering. Acta Biomater. 2009, 5, 406–418. [Google Scholar] [CrossRef]
- Bhat, S.; Lidgren, L.; Kumar, A. In Vitro Neo-Cartilage Formation on a Three-Dimensional Composite Polymeric Cryogel Matrix. Macromol. Biosci. 2013, 13, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Yoshitomi, H.; Fukumoto, K.; Danjo, S.; Yoshinaga, T.; Nishimata, H.; Yoshida, M. Toxic Chemical Cross-Linker-Free Cryosponges Made from Chitosan-Gluconic Acid Conjugate for Chondrocyte Culture. J. Chem. Eng. Jpn. 2017, 50, 142–148. [Google Scholar] [CrossRef]
- Li, P.; Jia, Z.; Wang, Q.; Tang, P.; Wang, M.; Wang, K.; Fang, J.; Zhao, C.; Ren, F.; Ge, X.; et al. A Resilient and Flexible Chitosan/Silk Cryogel Incorporated Ag/Sr Co-Doped Nanoscale Hydroxyapatite for Osteoinductivity and Antibacterial Properties. J. Mater. Chem. B 2018, 6, 7427–7438. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, A.; Kumar, A. Evaluating Potential of Tissue-Engineered Cryogels and Chondrocyte Derived Exosomes in Articular Cartilage Repair. Biotechnol. Bioeng. 2022, 119, 605–625. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-E.; Kim, S.-H.; Kim, H.D.; Yim, H.-G.; Bencherif, S.A.; Kim, T.-I.; Hwang, N.S. Extracellular Matrix-Based Cryogels for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Zhang, C.; Varghese, S. Poly(Ethylene Glycol) Cryogels as Potential Cell Scaffolds: Effect of Polymerization Conditions on Cryogel Microstructure and Properties. J. Mater. Chem. 2010, 20, 345–351. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The Potential of Polymeric Cryogels in Bioseparation. Bioseparation 2001, 10, 163–188. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, J.; Koh, R.H.; Shim, J.; Lee, J.-C.; Kim, T.-I.; Hwang, N.S. Enhanced Osteogenic Commitment of Human Mesenchymal Stem Cells on Polyethylene Glycol-Based Cryogel with Graphene Oxide Substrate. ACS Biomater. Sci. Eng. 2017, 3, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Thomas, J.; Sharma, A.; Panwar, V.; Kaushik, S.; Ghosh, D. A Bioinspired, Ice-Templated Multifunctional 3D Cryogel Composite Crosslinked through in Situ Reduction of GO Displayed Improved Mechanical, Osteogenic and Antimicrobial Properties. Mater. Sci. Eng. C 2021, 119, 111584. [Google Scholar] [CrossRef] [PubMed]
- Abueva, C.D.G.; Padalhin, A.R.; Min, Y.-K.; Lee, B.-T. Preformed Chitosan Cryogel-Biphasic Calcium Phosphate: A Potential Injectable Biocomposite for Pathologic Fracture. J. Biomater. Appl. 2015, 30, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Nimi, N.; Sivadas, V.P.; Lal, L.P.M.R.; Nair, P.D. Dual Crosslinked Pullulan-Gelatin Cryogel Scaffold for Chondrocyte-Mediated Cartilage Repair: Synthesis, Characterization and in Vitro Evaluation. Biomed. Mater. Bristol 2022, 17, 015001. [Google Scholar] [CrossRef] [PubMed]
- Bektas, E.I.; Gurel Pekozer, G.; Kök, F.N.; Torun Kose, G. Evaluation of Natural Gum-Based Cryogels for Soft Tissue Engineering. Carbohydr. Polym. 2021, 271, 118407. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.D.; Cengiz, U.; Arslan, Y.E.; Kiran, F.; Ceylan, A. From a Plant Secretion to the Promising Bone Grafts: Cryogels of Silicon-Integrated Quince Seed Mucilage by Microwave-Assisted Sol–Gel Reaction. J. Biosci. Bioeng. 2021, 131, 420–433. [Google Scholar] [CrossRef]
- Ak, F.; Oztoprak, Z.; Karakutuk, I.; Okay, O. Macroporous Silk Fibroin Cryogels. Biomacromolecules 2013, 14, 719–727. [Google Scholar] [CrossRef]
- Assegehegn, G.; Brito-de La Fuente, E.; Franco, J.M.; Gallegos, C. The Importance of Understanding the Freezing Step and Its Impact on Freeze-Drying Process Performance. J. Pharm. Sci. 2019, 108, 1378–1395. [Google Scholar] [CrossRef]
- Singh, D.; Tripathi, A.; Nayak, V.; Kumar, A. Proliferation of Chondrocytes on a 3-d Modelled Macroporous Poly(Hydroxyethyl Methacrylate)-Gelatin Cryogel. J. Biomater. Sci. Polym. Ed. 2011, 22, 1733–1751. [Google Scholar] [CrossRef]
- Yamamoto, T.; Randriantsilefisoa, R.; Sprecher, C.M.; D’Este, M. Fabrication of Collagen–Hyaluronic Acid Cryogels by Directional Freezing Mimicking Cartilage Arcade-like Structure. Biomolecules 2022, 12, 1809. [Google Scholar] [CrossRef]
- Odabas, S. Collagen-Carboxymethyl Cellulose-Tricalcium Phosphate Multi-Lamellar Cryogels for Tissue Engineering Applications: Production and Characterization. J. Bioact. Compat. Polym. 2016, 31, 411–422. [Google Scholar] [CrossRef]
- Joshi Navare, K.; Colombani, T.; Rezaeeyazdi, M.; Bassous, N.; Rana, D.; Webster, T.; Memic, A.; Bencherif, S.A. Needle-Injectable Microcomposite Cryogel Scaffolds with Antimicrobial Properties. Sci. Rep. 2020, 10, 18370. [Google Scholar] [CrossRef] [PubMed]
- Villard, P.; Rezaeeyazdi, M.; Colombani, T.; Joshi-Navare, K.; Rana, D.; Memic, A.; Bencherif, S.A. Autoclavable and Injectable Cryogels for Biomedical Applications. Adv. Healthc. Mater. 2019, 8, 1900679. [Google Scholar] [CrossRef]
- Kim, H.D.; Hong, X.; An, Y.-H.; Park, M.J.; Kim, D.-G.; Greene, A.K.; Padwa, B.L.; Hwang, N.S.; Lin, R.-Z.; Melero-Martin, J.M. A Biphasic Osteovascular Biomimetic Scaffold for Rapid and Self-Sustained Endochondral Ossification. Adv. Healthc. Mater. 2021, 10, e2100070. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Wang, J.; Wang, L.; Gao, C.; Li, B.; Wang, Y.; Wu, J.; Quan, C. Bioactive Gelatin Cryogels with BMP-2 Biomimetic Peptide and VEGF: A Potential Scaffold for Synergistically Induced Osteogenesis. Chin. Chem. Lett. 2022, 33, 1956–1962. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, S.S.; Sivashanmugam, A.; Kwon, J.; Kim, S.L.; Noh, M.Y.; Kwon, S.K.; Jayakumar, R.; Hwang, N.S. Bioglass-Incorporated Methacrylated Gelatin Cryogel for Regeneration of Bone Defects. Polymers 2018, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. 3D Bioprinting of Photocrosslinkable Hydrogel Constructs. J. Appl. Polym. Sci. 2015, 132, 42458. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Yang, J.; Wu, F.-H.; Cao, W.-B.; Zhou, T.; Wang, Z.-Y.; Tu, C.-X.; Gou, Z.-R.; Zhang, L.; Gao, C.-Y. A Macroporous Cryogel with Enhanced Mechanical Properties for Osteochondral Regeneration In Vivo. Chin. J. Polym. Sci. Engl. Ed. 2023, 41, 40–50. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, J.; Li, L.; Du, Z.; Cai, Q.; Yang, X. Hydroxyapatite Nanowire Composited Gelatin Cryogel with Improved Mechanical Properties and Cell Migration for Bone Regeneration. Biomed. Mater. Bristol 2019, 14, 045001. [Google Scholar] [CrossRef]
- Petrov, P.; Petrova, E.; Tsvetanov, C.B. UV-Assisted Synthesis of Super-Macroporous Polymer Hydrogels. Polymer 2009, 50, 1118–1123. [Google Scholar] [CrossRef]
- Öfkeli, F.; Demir, D.; Bölgen, N. Biomimetic Mineralization of Chitosan/Gelatin Cryogels and in Vivo Biocompatibility Assessments for Bone Tissue Engineering. J. Appl. Polym. Sci. 2021, 138, 50337. [Google Scholar] [CrossRef]
- Suner, S.S.; Demirci, S.; Yetiskin, B.; Fakhrullin, R.; Naumenko, E.; Okay, O.; Ayyala, R.S.; Sahiner, N. Cryogel Composites Based on Hyaluronic Acid and Halloysite Nanotubes as Scaffold for Tissue Engineering. Int. J. Biol. Macromol. 2019, 130, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Rogulska, O.Y.; Trufanova, N.A.; Petrenko, Y.A.; Repin, N.V.; Grischuk, V.P.; Ashukina, N.O.; Bondarenko, S.Y.; Ivanov, G.V.; Podorozhko, E.A.; Lozinsky, V.I.; et al. Generation of Bone Grafts Using Cryopreserved Mesenchymal Stromal Cells and Macroporous Collagen-Nanohydroxyapatite Cryogels. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2022, 110, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Teixeira, B.I.B.; Monteiro, F.J.M. Biomimetic Composite Scaffold With Phosphoserine Signaling for Bone Tissue Engineering Application. Front. Bioeng. Biotechnol. 2019, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.L.; Lee, S.S.; Kim, I.; Kwon, J.; Kwon, S.; Bae, T.; Hur, J.; Lee, H.; Hwang, N.S. Ectopic Transient Overexpression of OCT-4 Facilitates BMP4-Induced Osteogenic Transdifferentiation of Human Umbilical Vein Endothelial Cells. J. Tissue Eng. 2020, 11, 2041731420909208. [Google Scholar] [CrossRef]
- Olov, N.; Mirzadeh, H.; Moradi, R.; Rajabi, S.; Bagheri-Khoulenjani, S. Shape Memory Injectable Cryogel Based on Carboxymethyl Chitosan/Gelatin for Minimally Invasive Tissue Engineering: In Vitro and in Vivo Assays. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2022, 110, 2438–2451. [Google Scholar] [CrossRef] [PubMed]
- Tsung, L.H.; Chang, K.-H.; Chen, J.P. Osteogenesis of adipose-derived stem cells on three-dimensional, macroporous gelatin–hyaluronic acid cryogels. Biomed. Eng. Appl. Basis Commun. 2011, 23, 127–133. [Google Scholar] [CrossRef]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the Morphological, Mechanical and Degradation Properties of Scaffolds Comprising Collagen, Gelatin and Elastin for Use in Soft Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.C.G.; Shih, Y.-R.V.; Nakasaki, M.; Liu, M.; Varghese, S. Mineralized Biomaterials Mediated Repair of Bone Defects Through Endogenous Cells. Tissue Eng.-Part A 2018, 24, 1148–1156. [Google Scholar] [CrossRef]
- Odabas, S.; Feichtinger, G.A.; Korkusuz, P.; Inci, I.; Bilgic, E.; Yar, A.S.; Cavusoglu, T.; Menevse, S.; Vargel, I.; Piskin, E. Auricular Cartilage Repair Using Cryogel Scaffolds Loaded with BMP-7-Expressing Primary Chondrocytes. J. Tissue Eng. Regen. Med. 2013, 7, 831–840. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A. Bacterial Biopolymers and Genetically Engineered Biopolymers for Gel Systems Application. In Handbook of Biopolymer-Based Materials: From Blends and Composites to Gels and Complex Networks; Wiley: Hoboken, NJ, USA, 2013; pp. 87–107. [Google Scholar] [CrossRef]
- Katsen-Globa, A.; Meiser, I.; Petrenko, Y.A.; Ivanov, R.V.; Lozinsky, V.I.; Zimmermann, H.; Petrenko, A.Y. Towards Ready-to-Use 3-D Scaffolds for Regenerative Medicine: Adhesion-Based Cryopreservation of Human Mesenchymal Stem Cells Attached and Spread within Alginate-Gelatin Cryogel Scaffolds. J. Mater. Sci. Mater. Med. 2014, 25, 857–871. [Google Scholar] [CrossRef]
- Mishra, R.; Goel, S.K.; Gupta, K.C.; Kumar, A. Biocomposite Cryogels as Tissue-Engineered Biomaterials for Regeneration of Critical-Sized Cranial Bone Defects. Tissue Eng.-Part A 2014, 20, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Şarkaya, K.; Akıncıoğlu, G.; Akıncıoğlu, S. Investigation of Tribological Properties of HEMA-Based Cryogels as Potential Articular Cartilage Biomaterials. Polym.-Plast. Technol. Mater. 2022, 61, 1174–1190. [Google Scholar] [CrossRef]
- Ayaz, F.; Demir, D.; Bölgen, N. Injectable Chitosan Cryogel Microspheres with Biocompatible Properties on Mammalian Macrophages in Vitro. J. Mater. Sci. 2021, 56, 17268–17277. [Google Scholar] [CrossRef]
- Jain, E.; Srivastava, A.; Kumar, A. Macroporous Interpenetrating Cryogel Network of Poly(Acrylonitrile) and Gelatin for Biomedical Applications. J. Mater. Sci. Mater. Med. 2009, 20, S173–S179. [Google Scholar] [CrossRef]
- Yetiskin, B.; Akinci, C.; Okay, O. Cryogelation within Cryogels: Silk Fibroin Scaffolds with Single-, Double- and Triple-Network Structures. Polymer 2017, 128, 47–56. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, J.; Wu, X.; Lin, Y. Macroporous Double-Network Cryogels: Formation Mechanism, Enhanced Mechanical Strength and Temperature/pH Dual Sensitivity. Soft Matter 2011, 7, 4284. [Google Scholar] [CrossRef]
- Zhang, Y.; Leng, H.; Du, Z.; Huang, Y.; Liu, X.; Zhao, Z.; Zhang, X.; Cai, Q.; Yang, X. Efficient Regeneration of Rat Calvarial Defect with Gelatin-Hydroxyapatite Composite Cryogel. Biomed. Mater. Bristol 2020, 15, 065005. [Google Scholar] [CrossRef]
- Currey, J.D. Bones: Structure and Mechanics; Princeton University Press: Princeton, NJ, USA, 2002; ISBN 978-0-691-12804-7. [Google Scholar]
- Pal, S. Design of Artificial Human Joints & Organs; Springer US: Boston, MA, USA, 2014; ISBN 978-1-4614-6254-5. [Google Scholar]
- Lee, S.S.; Laganenka, L.; Du, X.; Hardt, W.-D.; Ferguson, S.J. Silicon Nitride, a Bioceramic for Bone Tissue Engineering: A Reinforced Cryogel System With Antibiofilm and Osteogenic Effects. Front. Bioeng. Biotechnol. 2021, 9, 794586. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Kuo, C.-Y.; Wong, C.-B.; Chien, Y.-M.; Chen, H.-A.; Chen, J.-P. Gelatin/Nanohyroxyapatite Cryogel Embedded Poly(Lactic-Co-Glycolic Acid)/Nanohydroxyapatite Microsphere Hybrid Scaffolds for Simultaneous Bone Regeneration and Load-Bearing. Polymers 2018, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhang, Y.; Zhang, L.; Huang, Y.; Zuo, D.; Cai, Q.; Yang, X. Comparative Study of Gelatin Cryogels Reinforced with Hydroxyapatites with Different Morphologies and Interfacial Bonding. Biomed. Mater. Bristol 2020, 15, 035012. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Tai, W.-C.; Ho, M.-H.; Chang, P.-C. Combination of a Biomolecule-Aided Biphasic Cryogel Scaffold with a Barrier Membrane Adhering PDGF-Encapsulated Nanofibers to Promote Periodontal Regeneration. J. Periodontal Res. 2020, 55, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Roether, J.; Bertels, S.; Oelschlaeger, C.; Bastmeyer, M.; Willenbacher, N. Microstructure, Local Viscoelasticity and Cell Culture Suitability of 3D Hybrid HA/Collagen Scaffolds. PLoS ONE 2018, 13, e0207397. [Google Scholar] [CrossRef]
- Liao, H.-T.; Shalumon, K.T.; Chang, K.-H.; Sheu, C.; Chen, J.-P. Investigation of Synergistic Effects of Inductive and Conductive Factors in Gelatin-Based Cryogels for Bone Tissue Engineering. J. Mater. Chem. B 2016, 4, 1827–1841. [Google Scholar] [CrossRef]
- Salgado, C.L.; Grenho, L.; Fernandes, M.H.; Colaço, B.J.; Monteiro, F.J. Biodegradation, Biocompatibility, and Osteoconduction Evaluation of Collagen-Nanohydroxyapatite Cryogels for Bone Tissue Regeneration. J. Biomed. Mater. Res.-Part A 2016, 104, 57–70. [Google Scholar] [CrossRef]
- Razavi, M.; Hu, S.; Thakor, A.S. A Collagen Based Cryogel Bioscaffold Coated with Nanostructured Polydopamine as a Platform for Mesenchymal Stem Cell Therapy. J. Biomed. Mater. Res.-Part A 2018, 106, 2213–2228. [Google Scholar] [CrossRef]
- Wu, S.; Ma, S.; Zhang, C.; Cao, G.; Wu, D.; Gao, C.; Lakshmanan, S. Cryogel Biocomposite Containing Chitosan-Gelatin/Cerium–Zinc Doped Hydroxyapatite for Bone Tissue Engineering. Saudi J. Biol. Sci. 2020, 27, 2638–2644. [Google Scholar] [CrossRef]
- Pandey, G.; Mittapelly, N.; Pant, A.; Sharma, S.; Singh, P.; Banala, V.T.; Trivedi, R.; Shukla, P.K.; Mishra, P.R. Dual Functioning Microspheres Embedded Crosslinked Gelatin Cryogels for Therapeutic Intervention in Osteomyelitis and Associated Bone Loss. Eur. J. Pharm. Sci. 2016, 91, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Gürer, B.; Yılmaz, C.; Yılmaz, Ş.N.; Çabuk, S.; Bölgen, N. A Novel Strategy for Cartilage Tissue Engineering: Collagenase-Loaded Cryogel Scaffolds in a Sheep Model. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 313–321. [Google Scholar] [CrossRef]
- Kemençe, N.; Bölgen, N. Gelatin- and Hydroxyapatite-Based Cryogels for Bone Tissue Engineering: Synthesis, Characterization, in Vitro and in Vivo Biocompatibility. J. Tissue Eng. Regen. Med. 2017, 11, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kwon, S.; Hwang, N.S.; Kang, B.-J. Clinical Application of Bone Morphogenetic Protein-2 Microcarriers Fabricated by the Cryopolymerization of Gelatin Methacrylate for the Treatment of Radial Fracture in Two Dogs. Vivo 2018, 32, 575–581. [Google Scholar] [CrossRef]
- Yuan, Z.; Yuan, X.; Zhao, Y.; Cai, Q.; Wang, Y.; Luo, R.; Yu, S.; Wang, Y.; Han, J.; Ge, L.; et al. Injectable GelMA Cryogel Microspheres for Modularized Cell Delivery and Potential Vascularized Bone Regeneration. Small 2021, 17, 2006596. [Google Scholar] [CrossRef]
- Di Muzio, L.; Cienzo, F.; Paolicelli, P.; Petralito, S.; Garzoli, S.; Brandelli, C.; Trilli, J.; Antonietta Casadei, M. A Convenient Strategy to Synthesize Highly Tunable Gelatin Methacryloyl with Very Low Gelation Temperature. Eur. Polym. J. 2021, 154, 110538. [Google Scholar] [CrossRef]
- Sakr, M.A.; Sakthivel, K.; Hossain, T.; Shin, S.R.; Siddiqua, S.; Kim, J.; Kim, K. Recent Trends in Gelatin Methacryloyl Nanocomposite Hydrogels for Tissue Engineering. J. Biomed. Mater. Res. A 2022, 110, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Young, A.T.; White, O.C.; Daniele, M.A. Rheological Properties of Coordinated Physical Gelation and Chemical Crosslinking in Gelatin Methacryloyl (GelMA) Hydrogels. Macromol. Biosci. 2020, 20, 2000183. [Google Scholar] [CrossRef]
- Nakamura, S.; Matsumoto, T.; Sasaki, J.; Egusa, H.; Lee, K.; Nakano, T.; Sohmura, T.; Nakahira, A. Effect of Calcium Ion Concentrations on Osteogenic Differentiation and Hematopoietic Stem Cell Niche-Related Protein Expression in Osteoblasts. Tissue Eng. Part A 2010, 16, 2467–2473. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Karakutuk, I.; Ak, F.; Okay, O. Diepoxide-Triggered Conformational Transition of Silk Fibroin: Formation of Hydrogels. Biomacromolecules 2012, 13, 1122–1128. [Google Scholar] [CrossRef]
- Volpi, N. Quality of Different Chondroitin Sulfate Preparations in Relation to Their Therapeutic Activity. J. Pharm. Pharmacol. 2009, 61, 1271–1280. [Google Scholar] [CrossRef]
- Volpi, N. Analytical Aspects of Pharmaceutical Grade Chondroitin Sulfates. J. Pharm. Sci. 2007, 96, 3168–3180. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Chondroitin Sulfate Safety and Quality. Molecules 2019, 24, 1447. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergen, G.; Zhunussova, M.; Mun, E.A.; Ramankulov, Y.; Ogay, V. Macroporous Cell-Laden Gelatin/Hyaluronic Acid/Chondroitin Sulfate Cryogels for Engineered Tissue Constructs. Gels 2022, 8, 590. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Chen, C.-H.; Hsiao, C.-Y.; Chen, J.-P. Incorporation of Chitosan in Biomimetic Gelatin/Chondroitin-6-Sulfate/Hyaluronan Cryogel for Cartilage Tissue Engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, B.; Colombani, T.; Joshi-Navare, K.; Mehta, S.; Kisiday, J.; Bencherif, S.A.; Bajpayee, A.G. Hyaluronic Acid-Based Shape-Memory Cryogel Scaffolds for Focal Cartilage Defect Repair. Tissue Eng.-Part A 2021, 27, 748–760. [Google Scholar] [CrossRef]
- Kao, H.-H.; Kuo, C.-Y.; Chen, K.-S.; Chen, J.-P. Preparation of Gelatin and Gelatin/Hyaluronic Acid Cryogel Scaffolds for the 3D Culture of Mesothelial Cells and Mesothelium Tissue Regeneration. Int. J. Mol. Sci. 2019, 20, 4527. [Google Scholar] [CrossRef]
- Gupta, A.; Bhat, S.; Jagdale, P.R.; Chaudhari, B.P.; Lidgren, L.; Gupta, K.C.; Kumar, A. Evaluation of Three-Dimensional Chitosan-Agarose-Gelatin Cryogel Scaffold for the Repair of Subchondral Cartilage Defects: An in Vivo Study in a Rabbit Model. Tissue Eng.-Part A 2014, 20, 3101–3111. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel Chitosan/Agarose/Hydroxyapatite Nanocomposite Scaffold for Bone Tissue Engineering Applications: Comprehensive Evaluation of Biocompatibility and Osteoinductivity with the Use of Osteoblasts and Mesenchymal Stem Cells. Int. J. Nanomedicine 2019, 14, 6615–6630. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Bölgen, N.; Yang, Y.; Korkusuz, P.; Güzel, E.; El Haj, A.J.; Pişkin, E. Three-Dimensional Ingrowth of Bone Cells within Biodegradable Cryogel Scaffolds in Bioreactors at Different Regimes. Tissue Eng.-Part A 2008, 14, 1743–1750. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-Based Foams and Aerogels: Processing, Properties, and Applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- Beck, S.; Bouchard, J.; Berry, R. Dispersibility in Water of Dried Nanocrystalline Cellulose. Biomacromolecules 2012, 13, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Souza, L.P.; Martins, T.M.M.; Lopes, J.H.; Mattos, B.D.; Mariano, M.; Pinheiro, I.F.; Valverde, T.M.; Livi, S.; Camilli, J.A.; et al. Nanocellulose/Bioactive Glass Cryogels as Scaffolds for Bone Regeneration. Nanoscale 2019, 11, 19842–19849. [Google Scholar] [CrossRef] [PubMed]
- Cal, F.; Sezgin Arslan, T.; Derkus, B.; Kiran, F.; Cengiz, U.; Arslan, Y.E. Synthesis of Silica-Based Boron-Incorporated Collagen/Human Hair Keratin Hybrid Cryogels with the Potential Bone Formation Capability. ACS Appl. Bio Mater. 2021, 4, 7266–7279. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Lopez-Cebral, R.; Sousa, R.O.; Alves, A.L.; Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Reis, R.L.; Silva, T.H. Marine Collagen-Chitosan-Fucoidan Cryogels as Cell-Laden Biocomposites Envisaging Tissue Engineering. Biomed. Mater. Bristol 2020, 15, 055030. [Google Scholar] [CrossRef] [PubMed]
- Ozurk, B.Y.; Inci, I.; Egri, S.; Ozturk, A.M.; Yetkin, H.; Goktas, G.; Elmas, C.; Piskin, E.; Erdogan, D. The Treatment of Segmental Bone Defects in Rabbit Tibiae with Vascular Endothelial Growth Factor (VEGF)-Loaded Gelatin/Hydroxyapatite “Cryogel” Scaffold. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, D.; Han, S.S. Fabrication of Three Dimensional Macroporous Matrix for Cardiac Regeneration. Int. J. Appl. Eng. Res. 2012, 7, 1226–1228. [Google Scholar]
- Shalumon, K.T.; Liao, H.-T.; Kuo, C.-Y.; Wong, C.-B.; Li, C.-J.; Mini, P.A.; Chen, J.-P. Rational Design of Gelatin/Nanohydroxyapatite Cryogel Scaffolds for Bone Regeneration by Introducing Chemical and Physical Cues to Enhance Osteogenesis of Bone Marrow Mesenchymal Stem Cells. Mater. Sci. Eng. C 2019, 104, 109855. [Google Scholar] [CrossRef]
- Bölgen, N.; Korkusuz, P.; Vargel, I.; Kiliç, E.; Güzel, E.; Çavuşoǧlu, T.; Uçkan, D.; Pişkin, E. Stem Cell Suspension Injected HEMA-Lactate-Dextran Cryogels for Regeneration of Critical Sized Bone Defects. Artif. Cells Nanomedicine Biotechnol. 2014, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Aguilar, M.R.; Fernández, M.D.M.; Gonzalo-Flores, S.; Villar-Rodil, S.; San Román, J.; Pişkin, E. Thermoresponsive Biodegradable HEMA-Lactate-Dextran-Co-NIPA Cryogels for Controlled Release of Simvastatin. Artif. Cells Nanomedicine Biotechnol. 2015, 43, 40–49. [Google Scholar] [CrossRef]
- Bölgen, N.; Vargel, I.; Korkusuz, P.; Güzel, E.; Plieva, F.; Galaev, I.; Matiasson, B.; Pişkin, E. Tissue Responses to Novel Tissue Engineering Biodegradable Cryogel Scaffolds: An Animal Model. J. Biomed. Mater. Res.-Part A 2009, 91, 60–68. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kuo, C.-Y.; Wang, Y.-J.; Chen, J.-P. Dual Function of Glucosamine in Gelatin/Hyaluronic Acid Cryogel to Modulate Scaffold Mechanical Properties and to Maintain Chondrogenic Phenotype for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2016, 17, 1957. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Palka, K.; Canal, C.; Kolodynska, D.; Przekora, A. Novel Synthesis Method Combining a Foaming Agent with Freeze-Drying to Obtain Hybrid Highly Macroporous Bone Scaffolds. J. Mater. Sci. Technol. 2020, 43, 52–63. [Google Scholar] [CrossRef]
- Bruns, J.; McBride-Gagyi, S.; Zustiak, S.P. Injectable and Cell-Adhesive Polyethylene Glycol Cryogel Scaffolds: Independent Control of Cryogel Microstructure and Composition. Macromol. Mater. Eng. 2018, 303, 1800298. [Google Scholar] [CrossRef]
- Dziubla, T.D.; Torjman, M.C.; Joseph, J.I.; Murphy-Tatum, M.; Lowman, A.M. Evaluation of Porous Networks of Poly(2-Hydroxyethyl Methacrylate) as Interfacial Drug Delivery Devices. Biomaterials 2001, 22, 2893–2899. [Google Scholar] [CrossRef] [PubMed]
- Casadio, Y.S.; Brown, D.H.; Chirila, T.V.; Kraatz, H.-B.; Baker, M.V. Biodegradation of Poly(2-Hydroxyethyl Methacrylate) (PHEMA) and Poly{(2-Hydroxyethyl Methacrylate)-Co-[Poly(Ethylene Glycol) Methyl Ether Methacrylate]} Hydrogels Containing Peptide-Based Cross-Linking Agents. Biomacromolecules 2010, 11, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jiao, H.; Xu, J.; Liu, Y.; Wei, S. Synthesis of Poly Hydroxypropyl Methacrylate Cryogel Incorporated with Zn/Ce Substituted Hydroxyapatite Nanoparticles for Rejuvenation of Femoral Fracture Treatment in a Rat Model. J. Photochem. Photobiol. B 2019, 201, 111651. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, X.; Quan, C.; Li, X.; Chen, C.; Kang, H.; Hu, W.; Jiang, Q.; Zhang, C. Poly(γ-Glutamic Acid) Induced Homogeneous Mineralization of the Poly(Ethylene Glycol)-Co-2-Hydroxyethyl Methacrylate Cryogel for Potential Application in Bone Tissue Engineering. RSC Adv. 2015, 5, 20227–20233. [Google Scholar] [CrossRef]
- Lytkina, D.N.; Fedorishin, D.A.; Kalachikova, P.M.; Plyaskina, A.A.; Babeshin, A.R.; Kurzina, I.A. Cryo-Structured Materials Based on Polyvinyl Alcohol and Hydroxyapatite for Osteogenesis. J. Funct. Biomater. 2021, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, R.; Lytkina, D.; Stepanova, K.; Kurzina, I. Synthesis of Biocompatible Composite Material Based on Cryogels of Polyvinyl Alcohol and Calcium Phosphates. Polymers 2022, 14, 3420. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Alvarez, V.A. Mechanical Properties of Polyvinylalcohol/Hydroxyapatite Cryogel as Potential Artificial Cartilage. J. Mech. Behav. Biomed. Mater. 2014, 34, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Eberlin, C.T.; Lu, T.; Neal, S.M.; Case, N.D.; McBride-Gagyi, S.H.; Sell, S.A. The Calcification Potential of Cryogel Scaffolds Incorporated with Various Forms of Hydroxyapatite for Bone Regeneration. Biomed. Mater. Bristol 2017, 12, 025005. [Google Scholar] [CrossRef] [PubMed]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-Hydroxyapatite and Its Applications in Preventive, Restorative and Regenerative Dentistry: A Review of Literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Cai, Y.; Ji, H.; Zhou, G.; Zhao, X.; Tang, R.; Zhang, M. In Vitro Effects of Nanophase Hydroxyapatite Particles on Proliferation and Osteogenic Differentiation of Bone Marrow-derived Mesenchymal Stem Cells. J. Biomed. Mater. Res. A 2009, 90A, 1083–1091. [Google Scholar] [CrossRef]
- Abdul Halim, N.A.; Hussein, M.Z.; Kandar, M.K. Nanomaterials-Upconverted Hydroxyapatite for Bone Tissue Engineering and a Platform for Drug Delivery. Int. J. Nanomedicine 2021, 16, 6477–6496. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, C.; Feng, X.; Wu, Z.; Lin, G.; Quan, C.; Chen, B.; Zhang, C. A Biomimicking Polymeric Cryogel Scaffold for Repair of Critical-Sized Cranial Defect in a Rat Model. Tissue Eng.-Part A 2019, 25, 1591–1604. [Google Scholar] [CrossRef]
- Covert, R.J.; Ott, R.D.; Ku, D.N. Friction Characteristics of a Potential Articular Cartilage Biomaterial. Wear 2003, 255, 1064–1068. [Google Scholar] [CrossRef]
- Świȩszkowski, W.; Ku, D.N.; Bersee, H.E.N.; Kurzydlowski, K.J. An Elastic Material for Cartilage Replacement in an Arthritic Shoulder Joint. Biomaterials 2006, 27, 1534–1541. [Google Scholar] [CrossRef]
- Mao, S.-H.; Chen, C.-H.; Chen, C.-T. Osteogenic Potential of Induced Pluripotent Stem Cells from Human Adipose-Derived Stem Cells. Stem Cell Res. Ther. 2019, 10, 303. [Google Scholar] [CrossRef]
- Salgado, C.L.; Barrias, C.C.; Monteiro, F.J.M. Clarifying the Tooth-Derived Stem Cells Behavior in a 3D Biomimetic Scaffold for Bone Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 724. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Zou, Y.; Zhu, J.; Liu, L.; Fan, Y. Preparation of Nanochitin Hydrogel with Adjustable Inter-Structure by Sequencial Genipin Crosslinking and Ice-Templating under Acid Condition. Int. J. Biol. Macromol. 2022, 221, 1022–1030. [Google Scholar] [CrossRef]
- Häussling, V.; Deninger, S.; Vidoni, L.; Rinderknecht, H.; Ruoß, M.; Arnscheidt, C.; Athanasopulu, K.; Kemkemer, R.; Nussler, A.K.; Ehnert, S. Impact of Four Protein Additives in Cryogels on Osteogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells. Bioengineering 2019, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Teotia, A.K.; Dienel, K.; Qayoom, I.; Van Bochove, B.; Gupta, S.; Partanen, J.; Seppälä, J. Improved Bone Regeneration in Rabbit Bone Defects Using 3d Printed Composite Scaffolds Functionalized with Osteoinductive Factors. ACS Appl. Mater. Interfaces 2020, 12, 48340–48356. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A. Effect of Plasma Polymerization on Physicochemical Properties of Biocomposite Cryogels Causing a Differential Behavior of Human Osteoblasts. J. Colloid Interface Sci. 2014, 431, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Ling, Y.; Deng, W.; Xue, J.; Sun, P.; Wang, D.-A. A Novel Cell Encapsulatable Cryogel (CECG) with Macro-Porous Structures and High Permeability: A Three-Dimensional Cell Culture Scaffold for Enhanced Cell Adhesion and Proliferation. Biomed. Mater. Bristol 2019, 14, 055006. [Google Scholar] [CrossRef]
- Offeddu, G.S.; Mela, I.; Jeggle, P.; Henderson, R.M.; Smoukov, S.K.; Oyen, M.L. Cartilage-like Electrostatic Stiffening of Responsive Cryogel Scaffolds. Sci. Rep. 2017, 7, srep42948. [Google Scholar] [CrossRef]
- Fassina, L.; Visai, L.; Magenes, G.; Schelfhout, J.; Bloise, N.; Riva, F.; Omes, C.; Avanzini, M.A.; Cusella De Angelis, M.G.; Benazzo, F.; et al. Ultrasound Stimulus to Enhance the Bone Regeneration Capability of Gelatin Cryogels. In Proceedings of the 35th Annual International Conference of the IEEE Eng Med Biol Soc, Osaka, Japan, 3–7 July, 2013; pp. 846–849. [Google Scholar] [CrossRef]
- Chen, T.-C.; Wong, C.-W.; Hsu, S.-H. Three-Dimensional Printing of Chitosan Cryogel as Injectable and Shape Recoverable Scaffolds. Carbohydr. Polym. 2022, 285, 119228. [Google Scholar] [CrossRef]
- Koh, R.H.; Kim, J.; Kim, S.H.L.; Hwang, N.S. RGD-Incorporated Biomimetic Cryogels for Hyaline Cartilage Regeneration. Biomed. Mater. Bristol 2022, 17, 024106. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Zhu, S.; Wei, X.; Zhou, N.; Liao, X.; Peng, Y.; Tang, Y.; Zhang, L.; Yang, X.; et al. Cryoprinting of Nanoparticle-Enhanced Injectable Hydrogel with Shape-Memory Properties. Mater. Des. 2022, 223, 111120. [Google Scholar] [CrossRef]
- Yang, X.; Meng, H.; Peng, J.; Xu, L.; Wang, Y.; Sun, X.; Zhao, Y.; Quan, Q.; Yu, W.; Chen, M.; et al. Construction of Microunits by Adipose-Derived Mesenchymal Stem Cells Laden with Porous Microcryogels for Repairing an Acute Achilles Tendon Rupture in a Rat Model. Int. J. Nanomedicine 2020, 15, 7155–7171. [Google Scholar] [CrossRef]
- Krasnov, M.S.; Shaikhaliev, A.I.; Korshakov, E.V.; Gasbanov, G.A.; Korgoloev, R.S.; Sinitskaya, E.S.; Sidorskii, E.V.; Yamskova, V.P.; Lozinsky, V.I. Changes in Rat Bone Tissue at the Site of the Defect In Vivo under the Effect of a Cryogenically Structured Albumin Sponge Containing a Bioregulator. Bull. Exp. Biol. Med. 2021, 170, 805–808. [Google Scholar] [CrossRef] [PubMed]








| Cross-Linking Method | Advantages | Disadvantages |
|---|---|---|
| Radical cross-linking | Relatively fast cross-linking reaction | Cito-toxicity of unreacted species Employable only with radical-sensitive polymers |
| GA | Employable on a wide range of bio-polymers containing nucleophiles on their backbone | Cito-toxicity of unreacted species |
| DVS | Employable on a wide range of bio-polymers containing nucleophiles on their backbone | Cito-toxicity of unreacted species |
| Carbodiimide coupling | Employable on a wide range of bio-polymers | Cito-toxicity of unreacted species |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carriero, V.C.; Di Muzio, L.; Petralito, S.; Casadei, M.A.; Paolicelli, P. Cryogel Scaffolds for Tissue-Engineering: Advances and Challenges for Effective Bone and Cartilage Regeneration. Gels 2023, 9, 979. https://doi.org/10.3390/gels9120979
Carriero VC, Di Muzio L, Petralito S, Casadei MA, Paolicelli P. Cryogel Scaffolds for Tissue-Engineering: Advances and Challenges for Effective Bone and Cartilage Regeneration. Gels. 2023; 9(12):979. https://doi.org/10.3390/gels9120979
Chicago/Turabian StyleCarriero, Vito Cosimo, Laura Di Muzio, Stefania Petralito, Maria Antonietta Casadei, and Patrizia Paolicelli. 2023. "Cryogel Scaffolds for Tissue-Engineering: Advances and Challenges for Effective Bone and Cartilage Regeneration" Gels 9, no. 12: 979. https://doi.org/10.3390/gels9120979