Hydrogel Polymer Electrolytes: Synthesis, Physicochemical Characterization and Application in Electrochemical Capacitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. FTIR–ATR Spectra Analysis
2.2. Electrolyte Sorption
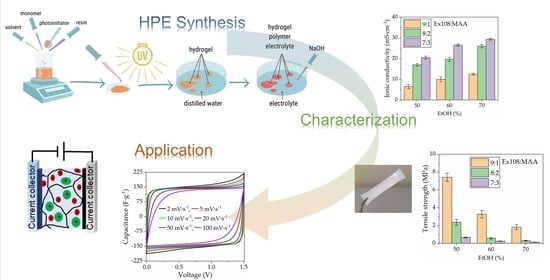
2.3. Ionic Conductivity
2.4. Mechanical Properties
2.5. Electrochemical Investigation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methodology
4.2.1. Hydrogel Preparation
4.2.2. FTIR-ATR Analysis
4.2.3. Electrolyte Sorption
4.2.4. Mechanical Properties
4.2.5. Ionic Conductivity
4.2.6. Electrochemical Investigation
Preparation of Electrodes and Electrochemical Capacitor
Electrochemical Capacitor Investigations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, X.; Jiang, C.; Sun, N.; Tan, D.; Li, Q.; Bi, S.; Song, J. Recent progress in multifunctional hydrogel-based supercapacitors. J. Sci. Adv. Mater. Devices 2021, 6, 338–350. [Google Scholar] [CrossRef]
- Chou, S.K.; Wei, Y.-M.; Yan, J. (Eds.) Handbook of Clean Energy Systems; Wiley: Chichester, UK, 2015; ISBN 9781118388587. [Google Scholar]
- Lin, Z.; Taberna, P.-L.; Simon, P. Electrochemical double layer capacitors: What is next beyond the corner? Curr. Opin. Electrochem. 2017, 6, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Kularatna, N.; Gunwardane, K. Energy Storage Devices for Renewable Energy-Based Systems: Rechargeable Batteries and Supercapacitors, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2021; ISBN 9780128231852. [Google Scholar]
- Zhang, F.; Wei, M.; Viswanathan, V.V.; Swart, B.; Shao, Y.; Wu, G.; Zhou, C. 3D printing technologies for electrochemical energy storage. Nano Energy 2017, 40, 418–431. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, K.C.S.; Vedhanarayanan, B. High-Performance Supercapacitors: A Comprehensive Review on Paradigm Shift of Conventional Energy Storage Devices. Batteries 2023, 9, 202. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, N.A.; Sampath, S.; Shukla, A.K. Hydrogel-polymer electrolytes for electrochemical capacitors: An overview. Energy Environ. Sci. 2009, 2, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Zito, R.; Ardebili, H. Energy Storage: A New Approach, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Scrivener Publishing LLC: Beverly, MA, USA, 2019; ISBN 9781119083962. [Google Scholar]
- Xia, L.; Yu, L.; Hu, D.; Chen, G.Z. Electrolytes for electrochemical energy storage. Mater. Chem. Front. 2017, 1, 584–618. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Roberts, A.J.; Danil de Namor, A.F.; Slade, R.C.T. Low temperature water based electrolytes for MnO2/carbon supercapacitors. Phys. Chem. Chem. Phys. 2013, 15, 3518–3526. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Li, C.; Zhang, H.; Sun, X.; Xu, N.; Ma, Y. Flexible solid-state supercapacitors based on a conducting polymer hydrogel with enhanced electrochemical performance. J. Mater. Chem. A 2014, 2, 19726–19732. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, X.; Dong, B.; Sun, N.; Zheng, L. Poly(ionic liquid) hydrogels exhibiting superior mechanical and electrochemical properties as flexible electrolytes. J. Mater. Chem. A 2016, 4, 1112–1118. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, R.; Liu, Y.; Wang, Y.; Wang, X.; Xiao, H.; Yuan, G. Concentrated hydrogel electrolyte for integrated supercapacitor with high capacitance at subzero temperature. Sci. China Chem. 2021, 64, 852–860. [Google Scholar] [CrossRef]
- Cho, Y.-G.; Hwang, C.; Cheong, D.S.; Kim, Y.-S.; Song, H.-K. Gel/Solid Polymer Electrolytes Characterized by In Situ Gelation or Polymerization for Electrochemical Energy Systems. Adv. Mater. 2019, 31, e1804909. [Google Scholar] [CrossRef]
- Camposeo, A.; Arkadii, A.; Romano, L.; D’Elia, F.; Fabbri, F.; Zussman, E.; Pisignano, D. Impact of size effects on photopolymerization and its optical monitoring in-situ. Addit. Manuf. 2022, 58, 103020. [Google Scholar] [CrossRef]
- Liu, S.; Borjigin, T.; Schmitt, M.; Morlet-Savary, F.; Xiao, P.; Lalevée, J. High-Performance Photoinitiating Systems for LED-Induced Photopolymerization. Polymers 2023, 15, 342. [Google Scholar] [CrossRef]
- Virya, A.; Abella, J.; Grindal, A.; Lian, K. Na2SO4—Polyacrylamide Electrolytes and Enabled Solid-State Electrochemical Capacitors. Batter. Supercaps 2020, 3, 194–200. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Zheng, H.; Zhao, L.; Liu, J.; Zou, C.; Dong, Z.; Li, X.; Wang, M.; Lin, Y. Cotton Fiber/PVA-Based Neutral Hydrogel with Al3+ as an Electrolyte Additive for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2023, 6, 644–656. [Google Scholar] [CrossRef]
- Rafidi, N.; Bashir, S.; Hina, M.; Gunalan, S.; Ramesh, S.; Ramesh, K. Renewable and soft dynamic supercapacitors based on poly (acrylamide) hydrogel electrolytes and porous carbon electrodes. Polym. Bull. 2023, 80, 1285–1302. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, Z.; Xue, Y.; Zhao, J.; Ji, J.; Wang, C.; Wu, Y. Ionic Liquid Cross-linked Poly(N -isopropylacrylamide) Hydrogel Electrolytes for Self-Protective Flexible Separator-Free Supercapacitors. Ind. Eng. Chem. Res. 2023, 62, 2741–2751. [Google Scholar] [CrossRef]
- Rosas, J.; Virya, A.; Wong, N.; Chua, J.; Lian, K. LiNO3-poly(vinyl alcohol) polymer electrolytes and its applications in electrochemical capacitors with extended operating temperatures. Solid State Ion. 2023, 399, 116272. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Lovestead, T.M.; Burdick, J.A.; Anseth, K.S.; Bowman, C.N. Understanding multivinyl monomer photopolymerization kinetics through modeling and GPC investigation of degradable networks. Polymer 2005, 46, 6226–6234. [Google Scholar] [CrossRef]
- Wang, Y.; Auad, M.L.; Beckingham, B.S. 3D printing flexible supercapacitors based on crosslinked poly (acrylic acid-co-vinylimidazole). Eng. Rep. 2023, e12650. [Google Scholar] [CrossRef]
- Prontera, C.T.; Gallo, N.; Giannuzzi, R.; Pugliese, M.; Primiceri, V.; Mariano, F.; Maggiore, A.; Gigli, G.; Sannino, A.; Salvatore, L.; et al. Collagen Membrane as Water-Based Gel Electrolyte for Electrochromic Devices. Gels 2023, 9, 310. [Google Scholar] [CrossRef]
- Sun, K.; Cui, S.; Gao, X.; Liu, X.; Lu, T.; Wei, H.; Peng, H.; Ma, G. Graphene oxide assisted triple network hydrogel electrolyte with high mechanical and temperature stability for self-healing supercapacitor. J. Energy Storage 2023, 61, 106658. [Google Scholar] [CrossRef]
- Hu, Q.; Cui, S.; Sun, K.; Shi, X.; Miao, W.; Wang, X.; Peng, H.; Ma, G. A redox-active dual-network hydrogel electrolyte for flexible supercapacitor. J. Energy Storage 2023, 68, 107815. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Qian, C.; Han, L.; Lu, Z.; Liu, L. Boosting PEDOT energy storage with a redox anthraquinone dopant for a flexible hydrogel supercapacitor at sub-zero temperatures. J. Mater. Chem. C 2023, 11, 7441–7450. [Google Scholar] [CrossRef]







| Formulation | Ex108 | MAA | EtOH |
|---|---|---|---|
| wt.% | |||
| Ex108:MAA = 9:1_EtOH50 | 45 | 50 | 5 |
| Ex108:MAA = 8:2_EtOH50 | 40 | 50 | 10 |
| Ex108:MAA = 7:3_EtOH50 | 35 | 50 | 15 |
| Ex108:MAA = 9:1_EtOH60 | 36 | 60 | 4 |
| Ex108:MAA = 8:2_EtOH60 | 32 | 60 | 8 |
| Ex108:MAA = 7:3_EtOH60 | 28 | 60 | 12 |
| Ex108:MAA = 9:1_EtOH70 | 27 | 70 | 3 |
| Ex108:MAA = 8:2_EtOH70 | 24 | 70 | 6 |
| Ex108:MAA = 7:3_EtOH70 | 21 | 70 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewski, P.; Żyła, W.; Kazimierczak, K.; Marcinkowska, A. Hydrogel Polymer Electrolytes: Synthesis, Physicochemical Characterization and Application in Electrochemical Capacitors. Gels 2023, 9, 527. https://doi.org/10.3390/gels9070527
Gajewski P, Żyła W, Kazimierczak K, Marcinkowska A. Hydrogel Polymer Electrolytes: Synthesis, Physicochemical Characterization and Application in Electrochemical Capacitors. Gels. 2023; 9(7):527. https://doi.org/10.3390/gels9070527
Chicago/Turabian StyleGajewski, Piotr, Wiktoria Żyła, Klaudia Kazimierczak, and Agnieszka Marcinkowska. 2023. "Hydrogel Polymer Electrolytes: Synthesis, Physicochemical Characterization and Application in Electrochemical Capacitors" Gels 9, no. 7: 527. https://doi.org/10.3390/gels9070527