Novel Gemini Surfactant for Binding Eu(III)-Polyoxometalate into Hydrogels and Polymer Latexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of 1,1′-(1,10-Decanediyl)-Bis [3-(Undec-10-en-1-yl)-Imidazolium] Bromide Salt, BIBr
2.2. Hydrogel Characterization
2.3. Swelling Behavior
2.4. Thermal Behavior of Hydogels
2.5. Rheological Behavior of Poly(Methyl Methacrylate) Latexes
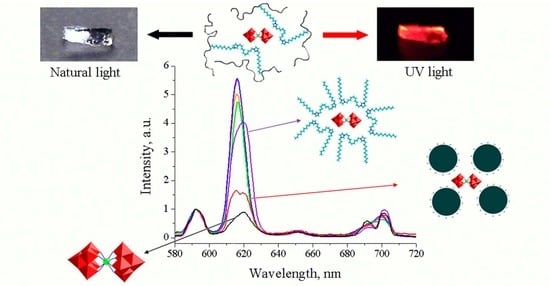
2.6. Luminescence of Eu(III) in Hydrogels and Latexes
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of 1,1′-(1,10-Decanediyl)-Bis [3-(Undec-10-en-1-yl)-Imidazolium] Bromide Salt, BIBr
4.3. Synthesis and Characterization of PAM Hydrogels Using BIBr as Crosslinking Agent
4.4. Emulsion Polymerization of PMMA Using BIBr as Surfmer
4.5. Luminescence of Europium (III) Probes in Different Environments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Madrida, K. Luminescence of lanthanide complexes: From fundamental to prospective approaches related to water- and molecular-stimuli. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Werts, M.H.V. Making sense of lanthanide luminescence. Sci. Prog. 2005, 88, 101–131. [Google Scholar] [CrossRef] [PubMed]
- Mahata, P.; Mondal, S.K.; Singha, D.K.; Majee, P. Luminescent rare-earth-based MOFs as optical sensors. Dalton Trans. 2017, 46, 301–328. [Google Scholar] [CrossRef] [PubMed]
- Kai, J.; Parra, D.F.; Brito, H.F. Polymer matrix sensitizing effect on photoluminescence of Eu3+-β-diketonate complex doped into poly-β-hydroxybutyrate (PHB) in film form. J. Mater. Chem. 2008, 18, 4549–4554. [Google Scholar] [CrossRef]
- Shahi, P.K.; Kumar, B.; Prakash, R.; Rai, S.B. Investigation of optical properties and energy transfer in Eu(III) and Tb(III) based composite compound dispersed in polar, non-polar solvents and polymer matrix. Mater. Res. Express 2019, 6, 046204. [Google Scholar] [CrossRef]
- De Jesus Morales Ramirez, A.; Murillo, A.G.; De Jesus Carrillo Romo, F.; Garcia Hernandez, M.; Palmerin, J.M.; Ruiz Guerrero, R. Preparation and scintillating properties of sol-gel Eu3+, Tb3+ co-doped Lu2O3 nanopowders. Int. J. Mol. Sci. 2011, 12, 6240–6254. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, J.D.; Bescher, E.P. Chemical routes in the synthesis of nanomaterials using the sol-gel process. Acc. Chem. Res. 2007, 40, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.-J.; Shen, Y.-T.; Liu, J.-J.; You, B.-J.; Ho, C.-H. Enhancing photovoltaic performance using broadband luminescent down-shifting by combining multiple species of Eu-doped silicate phosphors. Nanomaterials 2017, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Sato, M.; Tsukahara, Y. Fine control of red-green-blue photoluminescence in zeolites incorporated with rare-earth ions and a photosensitizer. Angew. Chemie. Int. Ed. 2006, 45, 1925–1928. [Google Scholar] [CrossRef] [PubMed]
- Gago, S.; Fernandes, J.A.; Rainho, J.P.; Sa Ferreira, R.A.; Pillinger, M.; Valente, A.A.; Santos, T.M.; Carlos, L.D.; Riberro-Claro, P.J.A.; Goncalves, I.S. Highly luminescent tris(β-diketonate)europium(III) complexes immobilized in a functionalized mesoporous silica. Chem. Mater. 2005, 17, 5077–5084. [Google Scholar] [CrossRef]
- Gong, Y.; Bai, F.; Yu, Z.; Bi, Y.; Xu, W.; Yu, L. PhotoluminescentEu-containing polyoxometalate/gemini surfactant hybrid nanoparticles for biological applications. RSC Adv. 2016, 6, 8601–8604. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Yan, Y.; Wu, L. Luminescent logic function of a surfactant-encapsulated polyoxometalate complex. Chem. Commun. 2006, 4575–4577. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Shao, Y.-F. Multicomponent hybrids with surfactant-encapsulated europium polyoxometalate covalently bonded ZnO and tunable luminescence. RSC Adv. 2014, 4, 3318–3325. [Google Scholar] [CrossRef]
- Wang, F.; Cao, S.; Men, J.; Lei, N.; Wang, R. Phtalocyanine-modified surfactant-encapsulated polyoxometalate and its self-assembly in solution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 125056. [Google Scholar] [CrossRef]
- Li, H.; Li, P.; Yang, Y.; Qi, W.; Sun, H.; Wu, L. Polystyrene latex by supramolecular encapsulation and miniemulsion polymerization. Macromol. Rapid Commun. 2008, 29, 431–436. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, M.; Xu, X.; Meng, K.; Yao, C.; Zhao, Y.; Sun, J.; Du, Y.; Yang, D. Multiresponsive supramolecular hydrogels based on nucleoside/lanthanide complex. ACS Appl. Mater. Interfaces 2019, 11, 47404–47412. [Google Scholar] [CrossRef]
- Green, M.; Harries, J.; Wakefield, G.; Taylor, R. The synthesis of silica nanospheres doped with polyoxometalates. J. Am. Chem. Soc. 2005, 127, 12812–12813. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ren, Q.; Lin, Z.; Wang, K.; Zhou, S.; Shi, Z.; Yin, J. Reversible stimuli-responsive luminescent polymers with adaptable mechanical properties based on europium-malonate complex. Polymer 2021, 214, 123259. [Google Scholar] [CrossRef]
- Gil-Kowalczyk, M.; Lyszczek, R.; Jusza, A.; Piramidowicz, R. Thermal, spectroscopy and luminescent characterization of hybrid PMMA/lanthanide complex materials. Materials 2021, 14, 3156. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, J.; Wang, Y.; Huanrong, L. Luminescent self-healing materials constructed via coordination between lanthanide ions and phenanthroline-tethered polymer chain. Dyes Pigment. 2022, 197, 109864. [Google Scholar] [CrossRef]
- Li, J.; Cui, G.; Bi, S.; Cui, X.; Li, Y.; Duan, Q.; Kakuchi, T.; Chen, Y. Eu3+- and Tb3+-based coordination complexes of poly(N-isopropyl,N-methylacrylamide-stat-N,N-dimethylacrylamide) copolymer: Synthesis, characterization and property. Polymers 2022, 14, 1815. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, L.; Zhang, Z.; Epaarachchi, J.; Li, L.; Hu, X.; Lu, C.; Xu, Z. Light-induced rare earth organic complex/shape-memory polymer composites with high strength and luminescence based on hydrogen bonding. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105525. [Google Scholar] [CrossRef]
- Guerrero-Hernandez, L.; Melendez-Ortiz, H.I.; Cortez-Mazatan, G.Y.; Vaillant-Sanchez, S.; Peralta-Rodriguez, R.D. Gemini and bicephalous surfactants: A review on their synthesis, micelle formation and uses. Int. J. Mol. Sci. 2022, 23, 1798. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; He, D.; Yu, Y.; Wu, S.; Dong, C.; Wang, H. Fluorescent sensitization of gemini surfactant micellar-hybridized supramolecular hydrogels. J. Lumin. 2017, 181, 8–13. [Google Scholar] [CrossRef]
- Pisarcik, M.; Polakovicova, M.; Markuliak, M.; Lukac, M.; Devinsky, F. Self-assembly properties of cationic gemini surfactants with biodegradable groups in the spacer. Molecules 2019, 24, 1481. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Malik, A.H.; Iyer, P.K. Highly precise detection, discrimination, and removal of anionic surfactants over the full pH range via cationic conjugated polymer: An efficient strategy to facilitate illicit-drug analysis. ACS Appl. Mater. Interfaces 2015, 7, 3189–3198. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Wang, F.; Zhang, Z.; Nie, H.; Li, N.; Cui, M. Synthesis, surface activity and aggregation behavior of Gemini imidazolium surfactants 1,3-bis-(3-alkylimidazolium-1-yl) propane bromide. Colloids Surf. A Physicochem. Eng. Asp. 2015, 467, 1–8. [Google Scholar] [CrossRef]
- Kamboj, R.; Singh, S.; Bhadani, A.; Kataria, H.; Kaur, G. Gemini imidazolium surfactants: Synthesis and their biophysiochemical study. Langmuir 2012, 28, 11969–11978. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Araghi, S.; Chen, D.-W.; Kohandel, M.; Sivaloganathan, S.; Foldvari, M. Tuning optimum transfection of gemini surfactant-phospholipid-DNA nanoparticles by validated theoretical modeling. Nanoscale 2019, 11, 1037–1046. [Google Scholar] [CrossRef]
- Zana, R.; Benrraou, M.; Rueff, R. Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 1991, 7, 1072–1075. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Zhuang, W.; Yao, M.; Pan, Y.; Chen, X. Spacer length effect on the aggregation behaviour of gemini surfactants in EAN. Coll. Polym. Sci. 2021, 299, 685–692. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Micutz, M.; Lungu, R.M.; Circu, V.; Ilis, M.; Staicu, T. Hydrogels obtained via γ-irradiation based on poly(acrylic acid) and its copolymers with 2-hydroxyethyl methacrylate. Appl. Sci. 2020, 10, 4960. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, S.; Xiang, Z.; Zhao, T.; Sun, D.; Zhang, G.; Chen, M.; Guo, K. Photoluminescent sensing vesicle platform self-asslembed by polyoxometalate and ionic-liquid-type imidazoliumgemini surfactants for the detection of Cr3+ and MnO4- ions. J. Coll. Interface Sci. 2019, 547, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ballardini, R.; Mulazzani, Q.G.; Venturi, M.; Bolletta, F.; Balzani, V. Photophysical characterization of the decatungstoeuropate(9-) anion. Inorg. Chem. 1984, 23, 300–305. [Google Scholar] [CrossRef]
- Li, H.; Qi, W.; Li, W.; Sun, H.; Bu, W.; Wu, L. A highly transparent and luminescent hybrid based on the copolymerization of surfactant-encapsulated polyoxometalate and methyl methacrylate. Adv. Mater. 2005, 17, 2688–2692. [Google Scholar] [CrossRef]
- Binnemans, K. Interpetration of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Zhang, S.; Tan, Y.; Sun, D.; Sun, P.; Cheng, X.; Xin, X. Self-assembly of europium-containing polyoxometalates/tetra-n-alkyl ammonium with enhanced emission for Cu2+ detection. ACS Omega 2018, 3, 14953–14961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bara, J.E.; Hatakeyama, E.S.; Weisenauer, B.R.; Zeng, X.; Noble, R.D.; Gin, D.L. Thermoplastic liquid crystal behaviour of geminiimidazolium-based ionic amphiphiles. Liq. Cryst. 2010, 37, 1587–1599. [Google Scholar] [CrossRef]
- Pana, A.; Ilis, M.; Micutz, M.; Dumitrascu, F.; Pasuk, I.; Circu, V. Liquid crystals based on silver carbine complexes derived from dimeric bis(imidazolium) bromide salts. RSC Adv. 2014, 4, 59491–59497. [Google Scholar] [CrossRef]
- Maximean, D.M.; Cîrcu, V.; Ganea, C.P. Dielectric properties of a bisimidazolium salt with dodecyl sulfate anion doped with carbon nanotubes. Beilstein J. Nanotechnol. 2018, 9, 164–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganea, C.P.; Cîrcu, V.; Manaila-Maximean, D. Effect of titanium oxide nanoparticles on the dielectric properties and ionic conductivity of a new smectic bis-imidazolium salt with dodecyl sulfate anion and cyanobiphenyl mesogenic groups. J. Mol. Liq. 2020, 317, 113939. [Google Scholar] [CrossRef]
- Ilincă, T.A.; Pasuk, I.; Cîrcu, V. Bis-imidazolium salts with alkyl sulfates as counterions: Synthesis and liquid crystalline properties. New J. Chem. 2017, 41, 11113–11124. [Google Scholar] [CrossRef]
- Sugeta, M.; Yamase, T. Crystal structure and luminescence site of Na9[EuW10O36].32H2O. Bull. Chem. Soc. Jpn. 1993, 66, 444–449. [Google Scholar] [CrossRef]
- Staicu, T.; Micutz, M.; Cristescu, G.; Leca, M. Temperature influence on surfactants adsorption onto poly(vinyl acetate) latices prepared via surfactant-free emulsion polymerization. Rev. Roum. Chim. 2008, 53, 481–487. [Google Scholar]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks. II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Adnadjevic, B.; Jovanovic, J. Hydrogel synthesis directed toward tissue engineering: Impact of reaction condition on structural parameters and macroscopic properties of xerogels. Int. J. Polym. Sci. 2011, 2011, 343062. [Google Scholar] [CrossRef]









| PAM2 | PAM4 | PAM6 | PAM10 | |
|---|---|---|---|---|
| SDeq (g/g) | 1070 | 306 | 58.2 | 18.6 |
| φ2.103 | 0.70 | 2.42 | 12.50 | 38.00 |
| Mc.10−5 (g/mol) | 37.10 | 5.52 | 0.35 | 0.05 |
| Hydrogel | k, min−n | n | R2 |
|---|---|---|---|
| PAM2 | 0.49 ± 0.05 | 0.78 ± 0.02 | 0.99078 |
| PAM4 | 0.51 ± 0.05 | 0.53 ± 0.02 | 0.97795 |
| PAM6 | 0.12 ± 0.01 | 0.59 ± 0.01 | 0.99363 |
| PAM10 | 0.10 ± 0.01 | 0.47 ± 0.02 | 0.95342 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micutz, M.; Circu, V.; Ilis, M.; Staicu, T. Novel Gemini Surfactant for Binding Eu(III)-Polyoxometalate into Hydrogels and Polymer Latexes. Gels 2022, 8, 786. https://doi.org/10.3390/gels8120786
Micutz M, Circu V, Ilis M, Staicu T. Novel Gemini Surfactant for Binding Eu(III)-Polyoxometalate into Hydrogels and Polymer Latexes. Gels. 2022; 8(12):786. https://doi.org/10.3390/gels8120786
Chicago/Turabian StyleMicutz, Marin, Viorel Circu, Monica Ilis, and Teodora Staicu. 2022. "Novel Gemini Surfactant for Binding Eu(III)-Polyoxometalate into Hydrogels and Polymer Latexes" Gels 8, no. 12: 786. https://doi.org/10.3390/gels8120786