Cryostructuring of Polymeric Systems: 63. Synthesis of Two Chemically Tanned Gelatin-Based Cryostructurates and Evaluation of Their Potential as Scaffolds for Culturing of Mammalian Cells †
Abstract
:1. Introduction
2. Results and Discussion
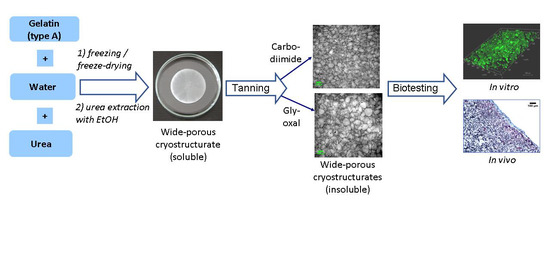
2.1. Preparation, Properties and Macroporous Morphology of Gelatin-Based Cryostructurates
2.2. Evaluation of the Gelatin-Based Cryostructurates as Scaffolds for Culturing of Mammalian Cells
2.2.1. Cytocompatibility of the EDC- and GXL-Tanned Gelatin-Based Cryostructurates
2.2.2. Proliferation and Liver Function Studies of HepG2 Cells upon Culturing in Gelatin-Based Cryostructurates
2.3. In Vivo Biocompatibility of Gelatin-Based Cryostructurates
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of the Cryogenically Structured Gelatin Scaffolds
4.3. Physico–Chemical Properties of the EDC- and GXL-Tanned Gelatin Cryostructurates
4.4. Microstructure of Gelatin-Based Cryostructurates
4.5. In Vitro Cell Culture
4.6. Live/Dead Assay
4.7. Prestoblue Assay
4.8. Liver Specific Functions Assays
4.9. In Vivo Studies
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jugur-Grodzinski, J. Polymers for tissue engineering, medical devices, and regenerative medicine: Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer scaffolds for biomaterials applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—State of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2012, 12, 107–124. [Google Scholar] [CrossRef]
- Rose, J.B.; Pacelli, S.; El Haj, A.J.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vlierberghe, S. Crosslinking strategies for porous gelatin scaffolds. J. Mater. Sci. 2016, 51, 4349–4357. [Google Scholar] [CrossRef]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as biomaterial for tissue engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Biomaterials in Tissue Engineering and Regenerative Medicine. From Basic Concepts to State of the Art Approaches; Bhaskar, B.; Rao, P.S.; Kasoju, N.; Nagarjuna, V.; Baadhe, R.R. (Eds.) Springer Nature Singapore Pte Ltd.: Singapore, 2021; p. 1039. ISBN 978-981-16-0001-2. [Google Scholar]
- Idumah, C.I. Recently emerging advancements in polymeric cryogels nanostructures and biomedical applications. Int. J. Polym. Mater. Biomater. 2022. [Google Scholar] [CrossRef]
- Blomeier, H.; Zhang, X.; Rives, C.; Brissova, M.; Hughes, E.; Baker, M.; Powers, A.C.; Kaufman, D.B.; Shear, L.D.; Lowe, W.L.J. Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation 2006, 82, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Okay, O. Macroporous copolymer networks. Progr. Polym. Sci. 2000, 25, 711–779. [Google Scholar] [CrossRef]
- Chung, C.; Burdick, J.A. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 2008, 60, 243–262. [Google Scholar] [CrossRef]
- Van Vierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Mahumane, G.D.; Kumar, P.; du Toit, L.C.; Choonara, Y.E.; Pillay, V. 3D scaffolds for brain tissue regeneration: Architectural challenges. Biomater. Sci. 2018, 6, 2812–2837. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and areas of implementation. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef]
- Zhang, H.; Cooper, A.I. Aligned porous structures by directional freezing. Adv. Mater. 2007, 19, 1529–1533. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Okay, O. (Ed.) Springer: Cham, Switzerland, 2014; p. 330. ISBN 978-3-319-05845-0. [Google Scholar]
- Lozinsky, V.I.; Okay, O. Basic principles of cryotropic gelation. Adv. Polym. Sci. 2014, 263, 49–102. [Google Scholar] [CrossRef]
- Reichelt, S. Introduction to macroporous cryogels. Methods Mol. Biol. 2015, 1286, 173–181. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, C.; Antoni, P.; Tomsia, A.P. Freeze casting for assembling bioinspired structural materials. Adv. Mater. 2017, 29, 1703155. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of polymer systems. 50. Cryogels and cryotropic gel-formation: Terms and definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of polymeric systems. 55. Retrospective view on the more than 40-years studies performed in the A.N. Nesmeyanov Institute of Organoelement Compounds with respect of the cryostructuring processes in polymeric systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jain, E.; Srivastava, A. Macroporous Polymeric Scaffolds for Tissue Engineering Applications. In Macroporous Polymers Production Properties and Biotechnological/Biomedical Applications; Mattiasson, B., Kumar, A., Galaev, I.Y., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 405–466. ISBN 978-1-4200-8461-0. [Google Scholar]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef] [PubMed]
- Gritsay, D.V.; Lebedinsky, A.S.; Ochenashko, O.V.; Rogul’skaya, E.Y.; Petrenko, Y.A.; Lozinsky, V.I.; Ivanov, R.V.; Petrenko, A.Y. Transplantation of cryopreserved fetal liver cells seeded into macroporous alginate-gelatin scaffolds in rats with liver failure. Russ. J. Transpl. Artif. Organs 2015, 17, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Supermacroporous Cryogels: Biomedical and Biotechnological Applications; Kumar, A. (Ed.) CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2016; p. 480. ISBN 978-1-4822-281-6. [Google Scholar]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Saylan, S.; Denizli, A. Supermacroporous composite cryogels in biomedical applications. Gels 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhshpour, M.; Idil, M.; Perçin, I.; Denizli, A. Biomedical applications of polymeric cryogels. Appl. Sci. 2019, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Klivenko, A.N.; Mussabaeva, B.K.; Gaisina, B.S.; Sabitova, A.N. Biocompatible cryogels: Preparation and application. Bull. Univ. Karaganda—Chem. 2021, 103, 4–20. [Google Scholar] [CrossRef]
- Savina, I.N.; Mohamed Zoughaib, M.; Abdulla, A.; Yergeshov, A.A. Design and assessment of biodegradable macroporous cryogels as advanced tissue engineering and drug carrying materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef]
- Çimen, D.; Özbek, M.A.; Bereli, N.; Mattiasson, B.; Denizli, A. Injectable cryogels in biomedicine. Gels 2021, 7, 38. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Andrabi, S.M.; Singh, A.; Majumder, S.; Kumar, A. Designing cryogels through cryostructuring of polymeric matrices for biomedical applications. Eur. Polym. J. 2021, 144, 110234. [Google Scholar] [CrossRef]
- Newland, B.; Long, K.R. Cryogel scaffolds: Soft and easy to use tools for neural tissue culture. Neural Regen. Res. 2022, 17, 1981–1983. [Google Scholar] [CrossRef] [PubMed]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterization. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I. A brief history of polymeric cryogels. Adv. Polym. Sci. 2014, 263, 1–48. [Google Scholar] [CrossRef]
- Okay, O.; Lozinsky, V.I. Synthesis, structure-property relationships of cryogels. Adv. Polym. Sci. 2014, 263, 103–157. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakoyama, F.; Achife, E.C.; Momoda, J.; Shimamura, K.; Monobe, K. Morphology of optically anisotropic agarose hydrogel prepared by directional freezing. Colloid Polym. Sci. 1990, 268, 552–558. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Bloch, K.O.; Vardi, P.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y. Cryostructuring of polymer systems. 29. Preparation and characterization of supermacroporous (spongy) agarose-based cryogels used as three-dimensional scaffolds for culturing insulin-producing cell aggregates. J. Appl. Polym. Sci. 2008, 108, 3046–3062. [Google Scholar] [CrossRef]
- Henisch, H.K. Crystal Growth in Gels; Dover Publications: New York, NY, USA, 1996; p. 112. ISBN 0486689158. [Google Scholar]
- Rogozhin, S.V.; Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Mamtsis, A.M.; Ivanova, S.A.; Shtil’man, M.I.; Korshak, V.V. Noncovalent cryostructurization in polymer systems. Dokl. Akad. Nauk SSSR 1984, 278, 129–133. (In Russian) [Google Scholar]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Effect of cryogenic treatment on the rheological properties of gelatin hydrogels. J. Bioact. Compat. Polym. 2010, 25, 498–512. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Processing of gelatin-based cryogels with improved thermomechanical resistance, pore size gradient, and high potential for sustainable protein drug release. J. Biomed. Mater. Res. Part A 2014, 103, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.; Volkova, N. Preparation and physicochemical characteristics of cryogel based on gelatin and oxidized dextran. J. Mater. Sci. 2014, 49, 4855–4868. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Kulakova, V.K.; Ivanov, R.V.; Petrenko, A.Y.; Rogulska, O.Y.; Petrenko, Y.A. Cryostructuring of polymer systems. 47. Preparation of wide porous gelatin-based cryostructurates in sterilizing organic media and assessment of the suitability of thus formed matrices as spongy scaffolds for 3D cell culturing. e-Polymers 2018, 18, 175–186. [Google Scholar] [CrossRef]
- He, Y.; Wang, C.; Wang, C.; Xiao, Y.; Lin, W. An overview on collagen and gelatin-based cryogels: Fabrication, classification, properties and biomedical applications. Polymers 2021, 13, 2299. [Google Scholar] [CrossRef]
- Koshy, S.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, porous and cell-responsive gelatin cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, R.V.; Eeman, M.; Rowshanravan, B.; Allan, I.U.; Savina, I.; Illsley, M.; Salmon, M.; James, S.E.; Mikhalovsky, S. The in vitro characterization of a gelatin scaffold, prepared by cryogelation and assessed in vivo as a dermal replacement in wound repair. Acta Biomater. 2014, 10, 3156–3166. [Google Scholar] [CrossRef]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R.G.S.V. Gelatin-based scaffold for tissue engineering—A review. Polym. Res. J. 2015, 9, 15–32. [Google Scholar]
- Liao, H.-T.; Shalumon, K.T.; Chang, K.-H.; Sheu, C.; Chen, J.-P. Investigation of synergistic effects of inductive and conductive factors in gelatin-based cryogels for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1827–1841. [Google Scholar] [CrossRef]
- Gandomani, M.G.; Lotfi, A.S.; Tamandani, D.K.; Arjmand, S.; Alizadeh, S. The enhancement of differentiating adipose derived mesenchymal stem cells toward hepatocyte like cells using gelatin cryogel scaffold. Biochem. Biophys. Res. Commun. 2017, 491, 1000–1006. [Google Scholar] [CrossRef]
- Han, M.-E.; Kang, B.J.; Kim, S.-H.; Kim, H.D.; Hwang, N.S. Gelatin-based extracellular matrix cryogels for cartilage tissue engineering. J. Ind. Eng. Chem. 2017, 45, 421–429. [Google Scholar] [CrossRef]
- Kao, H.-H.; Kuo, C.-Y.; Chen, K.-S.; Chen, J.-P. Preparation of gelatin and gelatin/hyaluronic acid cryogel scaffolds for the 3D culture of mesothelial cells and mesothelium tissue regeneration. Int. J. Mol. Sci. 2019, 20, 4527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, M.; Osada, E.; Takemura, Y.; Saito, H.; Kikuchi, E.; Nomura, K.; Sugimitsu, M.; Akiyama, N.; Kuwata, G.; Iketa, K.-I.; et al. Gelatin sponge as an anchorage for three-dimensional culture of colorectal cancer cells. Anticancer Res. 2021, 41, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Veis, A. The Macromolecular Chemistry of Gelatin; Academic Press: New York, NY, USA, 1964; p. 433. ISBN 978-0127154503. [Google Scholar]
- Petrenko, A.Y.; Khunov, Y.A.; Ivanov, E.N. Stem Cells. Properties and Possibilities of Clinical Applications; Press-Express: Lugansk, Ukraine, 2011; p. 368. ISBN 978-966-2327-11-3. [Google Scholar]
- Urrutia, D.N.; Caviedes, P.; Mardones, R.; Minguell, J.J.; Vega-Letter, A.M.; Jofre, C.M. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS ONE 2019, 14, e0213032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [Green Version]
- Sevastianov, V.I.; Basok, Y.B.; Kirsanova, L.A.; Grigoriev, A.M.; Kirillova, A.D.; Nemets, E.A.; Subbot, A.M.; Gautier, S.V. A comparison of the capacity of mesenchymal stromal cells for cartilage regeneration depending on collagen-based injectable biomimetic scaffold type. Life 2021, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Giannasi, C.; Niada, S.; Della Morte, E.; Casati, S.; Orioli, M.; Gualerzi, A.; Brini, A.M. Towards secretome standardization: Identifying key ingredients of MSC-derived therapeutic cocktail. Stem Cells Int. 2021, 2021, 3086122. [Google Scholar] [CrossRef]
- Kitano, H.; Kawabe, Y.; Kamihira, M. HepG2-based designer cells with heat-inducible enhanced liver functions. Cells 2022, 11, 1194. [Google Scholar] [CrossRef]
- Nakajima, N.; Ikada, Y. Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjugate Chem. 1995, 6, 123–130. [Google Scholar] [CrossRef]
- Roberts, J.D.; Caserio, M.C. Basic Principles of Organic Chemistry, 2nd ed.; Addison-Wesley: Boston, MA, USA, 1977; p. 1618. ISBN 9780805383294. [Google Scholar]
- Tanaka, T. Gels. In Encyclopedia of Polymer Science and Engineering; Mark, H.F., Bikales, N.M., Overberger, C.G., Menges, G., Eds.; A Wiley-Interscience Publisher: New York, NY, USA, 1987; Volume 7, pp. 514–531. ISBN 978-0471865193. [Google Scholar]
- Sergeev, G.B.; Batyuk, V.A. Reactions in frozen multicomponent systems. Russ. Chem. Rev. 1976, 45, 391–408. [Google Scholar] [CrossRef]
- Petrenko, Y.A.; Volkova, N.A.; Zhulikova, E.P.; Damshkaln, L.G.; Lozinsky, V.I.; Petrenko, A.Y. Choice of conditions for human bone marrow stromal cells seeding into polymer macroporous sponges. Biopolyners Cell 2008, 24, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Rodionov, I.A.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Shabatina, T.I.; Lozinsky, V.I. Cryostructuring of polymer systems. 44. Freeze-dried and then chemically cross linked wide porous cryostructurates based on serum albumin. e-Polymers 2017, 17, 263–274. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal stem cells for regenerative medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Richter., P. To Die or Not to Die: Cell Death in Biology and Disease. Int. J. Mol. Sci. 2022, 23, 6734. [Google Scholar] [CrossRef] [PubMed]
- Ruoss, M.; Vosough, M.; Konigsrainer, A.; Nadalin, S.; Wagner, S.; Sajadian, S.; Huber, D.; Heydari, Z.; Ehnert, S.; Hengstler, J.G.; et al. Towards improved hepatocyte cultures: Progress and limitations. Food Chem. Toxicol. 2020, 138, 111188. [Google Scholar] [CrossRef]
- Panwar, A.; Das, P.; Tan, L.P. 3D hepatic organoid-based advancements in liver tissue engineering. Bioengineering 2021, 8, 185. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The curious case of the HepG2 cell line: 40 years of expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef]
- Grigoriev, A.M.; Basok, Y.B.; Kirillova, A.D.; Kirsanova, L.A.; Shmerko, N.P.; Subbot, A.M.; Nemets, E.A.; Miloserdov, I.A.; Shagidulin, M.Y.; Sevastyanov, V.I. Experimental approaches to creating a tissue-specific matrix for a bioartificial liver. Russ. J. Transpl. Artif. Organs 2020, 22, 123–133. [Google Scholar] [CrossRef]
- Hammond, J.S.; Beckingham, I.J.; Shakesheff, K.M. Scaffolds for liver tissue engineering. Expert Rev. Med. Devices 2006, 3, 21–27. [Google Scholar] [CrossRef]
- Labour, M.N.; Le Guilcher, C.; Aid-Launais, R.; Samad, N.E.; Lanouar, S.; Simon-Yarza, T.; Letourneur, D. Development of 3D hepatic constructs within polysaccharide-based scaffolds with tunable properties. Int. J. Mol. Sci. 2020, 21, 3644. [Google Scholar] [CrossRef]
- Heydari, Z.; Najimi, M.; Mirzaei, H.; Shpichka, A.; Ruoss, M.; Farzaneh, Z.; Montazeri, L.; Piryaei, A.; Timashev, P.; Gramignoli, R.; et al. Tissue engineering in liver regenerative medicine: Insights into novel translational technologies. Cells 2020, 9, 304. [Google Scholar] [CrossRef] [Green Version]
- Ratner, B.D. The biocompatibility manifesto: Biocompatibility for the twenty-first century. J. Cardiovasc. Transl. Res. 2011, 4, 523–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Wagner, W.; Sakiyama-Elbert, S.; Zhang, G.; Yaszemski, M. (Eds.) Elsevier: London, UK, 2020; p. 1616. ISBN 9780128161371. [Google Scholar]
- Lozinsky, V.I.; Damshkaln, L.G.; Brown, C.R.T.; Norton, I.T. Study of cryostructuring of polymer systems. 19. On the nature of intermolecular links in the cryogels of locust bean gum. Polym. Int. 2000, 49, 1434–1443. [Google Scholar] [CrossRef]
- Tsvetkova, A.V.; Vakhrushev, I.V.; Basok, Y.B.; Grigor’ev, A.M.; Kirsanova, L.A.; Lupatov, A.Y.; Sevastianov, V.I.; Yarygin, K.N. Chondrogeneic potential of MSC from different sources in spheroid culture. Bull. Exp. Biol. Med. 2021, 170, 528–536. [Google Scholar] [CrossRef] [PubMed]







| Physico–Chemical Characteristics of Gelatin Sponges | |||
|---|---|---|---|
| Tanning Reagent | Stot (g of H2O (Bound + Capillary)/g of Dry Polymer) | Sw/w (g of Bound H2O/g of Dry Polymer) | Eapp (kPa) |
| EDC | 41.1 ± 3.3 | 2.48 ± 0.26 | 11.6 ± 0.5 |
| GXL | 48.6 ± 4.6 | 3.80 ± 0.28 | 5.18 ± 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozinsky, V.I.; Kulakova, V.K.; Grigoriev, A.M.; Podorozhko, E.A.; Kirsanova, L.A.; Kirillova, A.D.; Novikov, I.A.; Basok, Y.B.; Sevastianov, V.I. Cryostructuring of Polymeric Systems: 63. Synthesis of Two Chemically Tanned Gelatin-Based Cryostructurates and Evaluation of Their Potential as Scaffolds for Culturing of Mammalian Cells. Gels 2022, 8, 695. https://doi.org/10.3390/gels8110695
Lozinsky VI, Kulakova VK, Grigoriev AM, Podorozhko EA, Kirsanova LA, Kirillova AD, Novikov IA, Basok YB, Sevastianov VI. Cryostructuring of Polymeric Systems: 63. Synthesis of Two Chemically Tanned Gelatin-Based Cryostructurates and Evaluation of Their Potential as Scaffolds for Culturing of Mammalian Cells. Gels. 2022; 8(11):695. https://doi.org/10.3390/gels8110695
Chicago/Turabian StyleLozinsky, Vladimir I., Valentina K. Kulakova, Alexei M. Grigoriev, Elena A. Podorozhko, Ludmila A. Kirsanova, Aleksandra D. Kirillova, Ivan A. Novikov, Yulia B. Basok, and Viktor I. Sevastianov. 2022. "Cryostructuring of Polymeric Systems: 63. Synthesis of Two Chemically Tanned Gelatin-Based Cryostructurates and Evaluation of Their Potential as Scaffolds for Culturing of Mammalian Cells" Gels 8, no. 11: 695. https://doi.org/10.3390/gels8110695