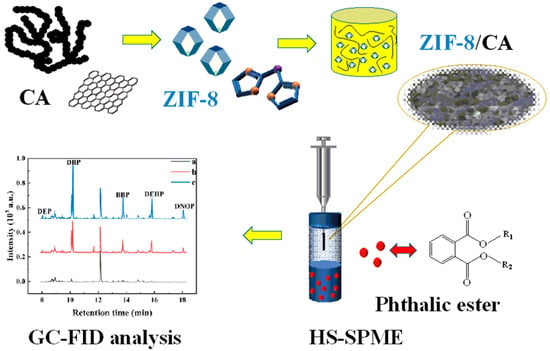
In Situ Synthetic ZIF-8/Carbon Aerogel Composites as Solid-Phase Microextraction Coating for the Detection of Phthalic Acid Esters in Water Samples
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Selection of the Best Hybrid Ratio of ZIF-8/CA
2.2. Characterization of ZIF-8/CA Coating
2.3. Optimization of the SPME Methods Using a ZIF-8/CA Coating
2.3.1. Extraction Time
2.3.2. Extraction Temperature
2.3.3. Stirring Rate
2.3.4. pH
2.3.5. Ionic Strength of the Solution
2.3.6. Sample Volume
2.3.7. Desorption Time
2.3.8. Desorption Temperature
2.4. Method Evaluation
2.5. Analysis of Real Samples
2.6. Comparison with Other Fibers in Extraction Performance
2.7. Durability of Extraction Device
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Instruments
4.3. Preparation of CA
4.4. Preparation of ZIF-8/CA
4.5. Preparation of ZIF-8/CA Coated SPME Fiber
4.6. HS-SPME Procedure
4.7. Real Sample Pretreatment
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Belardi, R.P.; Pawliszyn, J.B. The application of chemically modified fused silica fibers in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Qual. Res. J. 1989, 24, 179–191. [Google Scholar] [CrossRef]
- Wu, D.; Chen, X.; Liu, F.; Wu, J.; Zhao, G. A carbon dots-based coating for the determination of phthalate esters by solid-phase microextraction coupled gas chromatography in water samples. Microchem. J. 2020, 159, 105563. [Google Scholar] [CrossRef]
- Tian, T.; Wang, F.; Zhao, G. Magnesium/aluminum-layered double hydroxide modified with hydrogen peroxide as a novel fiber coating for solid-phase microextraction of phthalate esters in aqueous samples. Microchem. J. 2020, 153, 104510. [Google Scholar] [CrossRef]
- Mirzajani, R.; Kardani, F.; Ramezani, Z. Fabrication of UMCM-1 based monolithic and hollow fiber-Metal-organic framework deep eutectic solvents/molecularly imprinted polymers and their use in solid phase microextraction of phthalate esters in yogurt, water and edible oil by GC-FID. Food Chem. 2020, 314, 126179. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Cheng, L.; Sun, T.; Zhao, G.; Kan, X. Carbon aerogel as a solid-phase microextraction fiber coating for the extraction and detection of trace tetracycline residues in food by coupling with high-performance liquid chromatography. Anal. Methods 2021, 13, 381–389. [Google Scholar] [CrossRef]
- Sourya, S.; Firoozichahakb, A.; Nematollahic, D.; Alizadehc, S.; Kakaeia, H.; Abbasi, A. Needle-trap device packed with the MIL-100(Fe) metal–organic framework for the extraction of the airborne organochlorine pesticides. Microchem. J. 2021, 171, 106866. [Google Scholar] [CrossRef]
- Wu, D.; Liu, F.; Tian, T.; Wu, J.; Zhao, G. Copper ferrite nanoparticles as novel coating appropriated to solid-phase microextraction of phthalate esters from aqueous matrices. Microchem. J. 2021, 162, 105845. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Li, Z.; Wang, W.; Zang, X.; Wang, C.; Wang, Z. Solid phase microextraction of phthalic acid esters from vegetable oils using iron (III)-based metal-organic framework/graphene oxide coating. Food Chem. 2018, 263, 258–264. [Google Scholar] [CrossRef]
- Kong, J.; Zhu, F.; Huang, W.; He, H.; Hu, J.; Sun, C.; Xian, Q.; Yang, S. Sol-gel based metal-organic framework zeolite imidazolate framework-8 fibers for solid-phase microextraction of nitro polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbons in water samples. J. Chromatogr. A 2019, 1603, 92–101. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, S.; Wang, Q.; Lu, G.; Jiang, J.; Zhu, D. Zeolitic imidazolate framework-8 as sorbent of micro-solid-phase extraction to determine estrogens in environmental water samples. J. Chromatogr. A 2013, 1291, 27–32. [Google Scholar] [CrossRef]
- Dong, Z.; Cheng, L.; Sun, T.; Zhao, G.; Kan, X. Carboxylation modified meso-porous carbon aerogel templated by ionic liquid for solid-phase microextraction of trace tetracyclines residues using HPLC with UV detection. Microchim. Acta 2021, 188, 43. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, X.; Huang, C.; Chen, H.; Lu, Q.; Zhang, L. Metal-organic framework-derived nitrogen-doped carbon nanotube cages as efficient adsorbents for solid-phase microextraction of polychlorinated biphenyls. Anal. Chim. Acta 2020, 1095, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, Q.; Han, Q.; Wan, W.; Ding, M. Metal-organic frameworks@graphene hybrid aerogels for solid-phase extraction of non-steroidal anti-inflammatory drugs and selective enrichment of proteins. Analyst 2016, 141, 4219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Zou, Y.; Yang, G. Distribution and ecotoxicological state of phthalate esters in the sea-surface microlayer, seawater and sediment of the Bohai Sea and the Yellow Sea. Environ. Pollut. 2018, 240, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, X.; Peng, G.; Ning, W.; Lai, Z.; He, J.; Zheng, Z. Distribution and risk assessment of phthalates in water and sediment of the Pearl River Delta. Environ. Sci. Pollut. Res. 2020, 27, 12550–12565. [Google Scholar]
- Radke, E.; Braun, J.; Nachman, R.; Cooper, G. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ. Int. 2020, 137, 105408. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, D.; Mei, G.; Hong, X.; Wu, J.; Zheng, J.; Pang, J.; Yan, Z. Preparation of konjac glucomannan-based zeolitic imidazolate framework-8 composite aerogels with high adsorptive capacity of ciprofloxacin from water. Colloids Surf. A 2018, 544, 187–195. [Google Scholar] [CrossRef]
- Bo, S.; Ren, W.; Lei, C.; Xie, Y.; Cai, Y.; Wang, S.; Gao, J.; Ni, Q.; Yao, J. Flexible and porous cellulose aerogels/zeolitic imidazolate framework (ZIF-8) hybrids for adsorption removal of Cr (IV) from water. J. Solid State Chem. 2018, 262, 135–141. [Google Scholar] [CrossRef]
- Yao, J.; Chen, R.; Wang, K.; Wang, H. Direct synthesis of zeolitic imidazolate framework-8/chitosan composites in chitosan hydrogels. Microporous Mesoporous Mater. 2013, 165, 200–204. [Google Scholar] [CrossRef]
- Fang, X.; Yuan, X.; Song, Y.; Wang, J.; Lin, M. Cooperative lone pair-π and coordination interactions in naphthalene diimide coordination networks. CrystEngComm 2014, 16, 9090–9095. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Shu, Y.; Ma, L.; Zhang, X.; Ding, Y. Anchoring polysulfides in hierarchical porous carbon aerogel via electric-field-responsive switch for lithium sulfur battery. Electrochim. Acta 2019, 293, 458–465. [Google Scholar] [CrossRef]
- Park, S.; Choi, K.; Lee, S.; Oh, I.; Park, S.; Park, H. CNT branching of three-dimensional steam-activated graphene hybrid frameworks for excellent rate and cyclic capabilities to store lithium ions. Carbon 2017, 116, 500–509. [Google Scholar] [CrossRef]
- Tian, F.; Cerro, A.; Mosier, A.; Wayment-Steele, H.; Shine, R.; Park, A.; Webster, E.; Johnson, L.; Johal, M.; Benz, L. Surface and stability characterization of a nanoporous ZIF-8 thin film. J. Phys. Chem. C 2014, 118, 14449–14456. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, J.; Li, J.; Chen, D.; Kong, Y.; Chu, F.; Tao, Y.; Liu, M. Crosslinking graphene oxide into robust 3D porous N-doped graphene. Adv. Mater. 2015, 27, 5171–5175. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, J.; Liu, D.; Kang, R.; Yu, G.; Deng, S. Synthesis of mixed-linker Zr-MOFs for emerging contaminant adsorption and photodegradation under visible light. Chem. Eng. J. 2019, 378, 122118. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Yi, X.; Zhao, X.; Liu, B.; Liu, X. Nitrogen-doped hierarchical porous carbon derived from ZIF-8 supported on carbon aerogels with advanced performance for supercapacitor. Appl. Surf. Sci. 2020, 507, 145166. [Google Scholar] [CrossRef]
- Zhu, F.; Guo, J.; Zeng, F.; Fu, R.; Wu, D.; Luan, T.; Tong, Y.; Lu, T.; Ouyang, G. Preparation and characterization of porous carbon material-coated solid-phase microextraction metal fibers. J. Chromatogr. A 2010, 1217, 7848–7854. [Google Scholar] [CrossRef]
- Lu, L.; Wang, J.; Chen, B. Adsorption and desorption of phthalic acid esters on graphene oxide and reduced graphene oxide as affected by humic acid. Environ. Pollut. 2018, 232, 505–513. [Google Scholar] [CrossRef]
- Meng, W.; Liu, L.; Wang, X.; Zhao, R.; Wang, M.; Lin, J. Polyphenylene core-conjugated microporous polymer coating for highly sensitive solid-phase microextraction of polar phenol compounds in water samples. Anal. Chim. Acta 2018, 1015, 27–34. [Google Scholar] [CrossRef]
- Zou, J.; Song, X.; Ji, J.; Xu, W.; Chen, J.; Jiang, Y.; Wang, Y.; Chen, X. Polypyrrole/graphene composite-coated fiber for the solid-phase microextraction of phenols. J. Sep. Sci. 2011, 34, 2765–2772. [Google Scholar] [CrossRef]
- Pérez-Outeiral, J.; Millán, E.; Garcia-Arrona, R. Determination of phthalates in food simulants and liquid samples using ultrasound-assisted dispersive liquid-liquid microextraction followed by solidification of floating organic drop. Food Control 2016, 62, 171–177. [Google Scholar] [CrossRef]
- He, J.; Lv, R.; Zhu, J.; Lu, K. Selective solid-phase extraction of dibutyl phthalate from soybean milk using molecular imprinted polymers. Anal. Chim. Acta 2010, 661, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, H.; Yamini, Y.; Moradi, M.; Asl, Y.A. Determination of phthalate esters in drinking water and edible vegetable oil samples by headspace solid phase microextraction using graphene/polyvinylchloride nanocomposite coated fiber coupled to gas chromatography-flame ionization detector. J. Chromatogr. A 2016, 1465, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Eskandarpour, N.; Sereshti, H. Electrospun polycaprolactam-manganese oxide fiber for headspace-solid phase microextraction of phthalate esters in water samples. Chemosphere 2018, 191, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Jin, Y.; Zhao, G.; Li, M.; Li, D. Electrosorption-promoted photodegradation of opaque wastewater on a novel TiO2/carbon aerogel electrode. Environ. Sci. Technol. 2010, 44, 1780–1785. [Google Scholar] [CrossRef] [PubMed]





| Analytes | LR (μg L−1) | Regression Equation | R2 | EF | LODs (μg L−1) | LOQs (μg L−1) | RSDs (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| One Fiber Inter-Day (n = 5) | One Fiber Intra-Day (n = 5) | Fiber to Fiber (n = 3) | |||||||
| DEP | 0.5–1000 | y = 1350.2x + 1420.4 | 0.9985 | 252 | 0.32 | 1.05 | 5.97 | 3.74 | 5.66 |
| DBP | 0.2–500 | y = 16,117x + 82,578 | 0.9996 | 642 | 0.17 | 0.58 | 5.02 | 7.06 | 9.50 |
| BBP | 0.5–1000 | y = 5768.1x + 102,527 | 0.9959 | 329 | 0.22 | 0.72 | 10.57 | 5.71 | 12.11 |
| DEHP | 1–250 | y = 6136.4x + 36,030 | 0.9991 | 544 | 0.48 | 1.60 | 7.14 | 3.50 | 8.48 |
| DNOP | 0.5–500 | y = 2141.6x + 22,282 | 0.9946 | 641 | 0.30 | 0.99 | 7.02 | 8.16 | 9.13 |
| Analytes | River Water Samples | Bottled Water Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| Spiking (μg L−1) | Found (μg L−1) | Recovery (%) | RSD (%, n = 3) | Spiking (μg L−1) | Found (μg L−1) | Recovery (%) | RSD (%, n = 3) | |
| DEP | 0 | ND * | - | - | 0 | ND | - | - |
| 50 | 42.21 | 84.42 | 4.98 | 50 | 42.27 | 84.54 | 3.66 | |
| 100 | 80.71 | 80.71 | 10.97 | 100 | 83.83 | 83.83 | 3.24 | |
| DBP | 0 | ND | - | - | 0 | ND | - | - |
| 50 | 40.58 | 81.16 | 12.31 | 50 | 40.41 | 80.82 | 7.70 | |
| 100 | 83.41 | 83.41 | 7.31 | 100 | 81.14 | 81.14 | 9.32 | |
| BBP | 0 | ND | - | - | 0 | ND | - | - |
| 50 | 52.76 | 105.52 | 9.71 | 50 | 56.79 | 113.58 | 4.00 | |
| 100 | 117.20 | 117.20 | 10.63 | 100 | 97.97 | 97.97 | 4.22 | |
| DEHP | 0 | ND | - | - | 0 | ND | - | - |
| 50 | 52.80 | 105.60 | 11.54 | 50 | 52.65 | 105.30 | 4.07 | |
| 100 | 108.28 | 108.28 | 11.62 | 100 | 102.24 | 102.24 | 8.65 | |
| DNOP | 0 | ND | - | - | 0 | ND | - | - |
| 50 | 58.57 | 117.14 | 5.81 | 50 | 56.25 | 112.50 | 8.13 | |
| 100 | 114.47 | 114.47 | 4.00 | 100 | 117.50 | 117.50 | 15.30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.-M.; Zhang, P.; Sun, T.; Xia, Q.; Wu, J.-F.; Zhao, G.-C. In Situ Synthetic ZIF-8/Carbon Aerogel Composites as Solid-Phase Microextraction Coating for the Detection of Phthalic Acid Esters in Water Samples. Gels 2022, 8, 610. https://doi.org/10.3390/gels8100610
Dong Z-M, Zhang P, Sun T, Xia Q, Wu J-F, Zhao G-C. In Situ Synthetic ZIF-8/Carbon Aerogel Composites as Solid-Phase Microextraction Coating for the Detection of Phthalic Acid Esters in Water Samples. Gels. 2022; 8(10):610. https://doi.org/10.3390/gels8100610
Chicago/Turabian StyleDong, Zong-Mu, Peiyi Zhang, Tong Sun, Qian Xia, Jian-Feng Wu, and Guang-Chao Zhao. 2022. "In Situ Synthetic ZIF-8/Carbon Aerogel Composites as Solid-Phase Microextraction Coating for the Detection of Phthalic Acid Esters in Water Samples" Gels 8, no. 10: 610. https://doi.org/10.3390/gels8100610