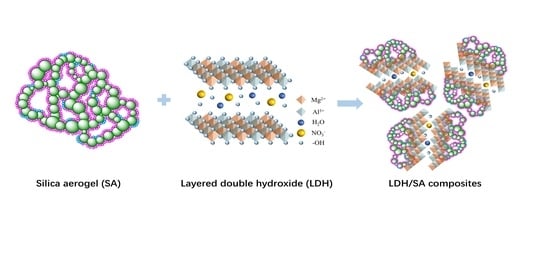
In-Situ Synthesis of Layered Double Hydroxide/Silica Aerogel Composite and Its Thermal Safety Characteristics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phase Composition of MgAl-LDH and LDH/SA Composites
2.2. Microstructures of MgAl-LDH and LDH/SA
2.3. Pore Structure
2.4. Density, Porosity and Thermal Conductivity
2.5. Surface Properties and Hydrophobicity
2.6. Thermal Safety Characteristics
2.6.1. Composition Analysis of LDH/SA under Heat Treatment
2.6.2. Thermal Analysis
2.6.3. Gross Calorific Value
3. Conclusions
4. Materials and Methods
4.1. Raw Materials
4.2. Preparation of MgAl-LDH
4.3. Preparation of LDH/SA Composites
4.4. Methods of Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels—Airy Materials: Chemistry, Structure, and Properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive Manufacturing of Silica Aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward Aerogel Based Thermal Superinsulation in Buildings: A Comprehensive Review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Huber, L.; Wu, X.; Huang, S.; Zhang, Y.; Huang, R.; Liu, Q. Reducing the Thermal Hazard of Hydrophobic Silica Aerogels by Using Dimethyldichlorosilane as Modifier. J. Sol-Gel Sci. Technol. 2020, 93, 111–122. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; He, S.; Shi, X.; Yang, H. Characteristics of Ambient-Pressure-Dried Aerogels Synthesized via Different Surface Modification Methods. J. Sol-Gel Sci. Technol. 2015, 76, 138–149. [Google Scholar] [CrossRef]
- Parale, V.G.; Jung, H.-N.-R.; Han, W.; Lee, K.-Y.; Mahadik, D.B.; Cho, H.H.; Park, H.-H. Improvement in the High Temperature Thermal Insulation Performance of Y2O3 Opacified Silica Aerogels. J. Alloys Compd. 2017, 727, 871–878. [Google Scholar] [CrossRef]
- Baskaran, T.; Kumaravel, R.; Christopher, J.; Sakthivel, A. Silicate Anion Intercalated Cobalt-Aluminium Hydrotalcite (CoAl-HT-Si): A Potential Catalyst for Alcohol Oxidation. Rsc Adv. 2014, 4, 11188–11196. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Stoeckelhuber, K.W.; Wagenknecht, U.; Heinrich, G. Advances in Layered Double Hydroxide (LDH)-Based Elastomer Composites. Prog. Polym. Sci. 2014, 39, 594–626. [Google Scholar] [CrossRef]
- El Khattabi, E.H.; Mourid, E.H.; Belaaouad, S.; Naimi, Y. Layered Double Hydroxide Nanomaterial as Highly Efficient Adsorbent and Its Recycling after Removal of a Carcinogenic Tartrazine Dye from Wastewater. Biointerface Res. Appl. Chem. 2022, 12, 7725–7740. [Google Scholar] [CrossRef]
- Lin, Y.; Ruiyi, L.; Zaijun, L.; Junkang, L.; Yinjun, F.; Guangli, W.; Zhiguo, G. Three-Dimensional Activated Reduced Graphene Oxide Nanocup/Nickel Aluminum Layered Double Hydroxides Composite with Super High Electrochemical and Capacitance Performances. Electrochim. Acta 2013, 95, 146–154. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khosravi, F.; Mossayebi, Z.; Saedi Ardahaei, A.; Morshedi Dehaghi, F.; Khorasani, M.; Neisiany, R.E.; Das, O.; Marani, A.; Mensah, R.A.; et al. The Flame Retardancy of Polyethylene Composites: From Fundamental Concepts to Nanocomposites. Molecules 2020, 25, 5157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, Y.; Zhang, Z.; Wang, Q. Preparation of Ammonium Polyphosphate and Dye Co-Intercalated LDH/Polypropylene Composites with Enhanced Flame Retardant and UV Resistance Properties. Chemosphere 2021, 277, 130370. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Xu, S.; Tian, X.-Y.; Zeng, H.-Y.; Hu, J.; Guo, Y.-H.; Jian, J. Renewable Lignin-Based Surfactant Modified Layered Double Hydroxide and Its Application in Polypropylene as Flame Retardant and Smoke Suppression. Int. J. Biol. Macromol. 2021, 178, 580–590. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Lin, Y.; Li, R.; Zhang, F. Layered Double Hydroxides Used as Flame Retardant for Engineering Plastic Acrylonitrile-Butadiene-Styrene (ABS). J. Phys. Chem. Solids 2012, 73, 1514–1517. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered Double Hydroxides: A Brief Review from Fundamentals to Application as Evolving Biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of Layered Double Hydroxides for Removal of Oxyanions: A Review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, G.; Zhang, R.; Chen, C.; Wan, C.; Cai, Y.; Yao, J. Synergistic Effects of Intercalation of Layered Double Hydroxide with Phytic Acid-Grafted β-Cyclodextrin on the Flame Retardancy of Polypropylene. Mater. Today Commun. 2022, 32, 103904. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Gao, S.; Lu, Y.; Liu, J.; Zhu, H. Two-Dimensional Layered Double Hydroxides Nanoplatelets Assembled in Situ on SiO2 Nanoparticles for High-Performing Hydrogenated Nitrile Butadiene Rubber. Compos. Sci. Technol. 2019, 182, 107742. [Google Scholar] [CrossRef]
- Lv, S.; Kong, X.; Wang, L.; Zhang, F.; Lei, X. Flame-Retardant and Smoke-Suppressing Wood Obtained by the in Situ Growth of a Hydrotalcite-like Compound on the Inner Surfaces of Vessels. New J. Chem. 2019, 43, 16359–16366. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z. Intercalation of Sulfate Anions into a Zn-Al Layered Double Hydroxide: Their Synthesis and Application in Zn-Ni Secondary Batteries. RSC Adv. 2016, 6, 68584–68591. [Google Scholar] [CrossRef]
- Zhao, Y. Mechanism of Pore Formation and Structural Characterization for Mesoporous Mg-Al Composite Oxides. Sci. China Ser. B 2002, 45, 37. [Google Scholar] [CrossRef]
- Yasaei, M.; Khakbiz, M.; Zamanian, A.; Ghasemi, E. Synthesis and Characterization of Zn/Al-LDH@SiO2 Nanohybrid: Intercalation and Release Behavior of Vitamin C. Mater. Sci. Eng. C 2019, 103, 109816. [Google Scholar] [CrossRef]
- Long, J.; Yang, Z.; Huang, J.; Zeng, X. Self-Assembly of Exfoliated Layered Double Hydroxide and Graphene Nanosheets for Electrochemical Energy Storage in Zinc/Nickel Secondary Batteries. J. Power Source 2017, 359, 111–118. [Google Scholar] [CrossRef]
- Rojas, F.; Kornhauser, I.; Felipe, C.; Esparza, J.M.; Cordero, S.; Domínguez, A.; Riccardo, J.L. Capillary Condensation in Heterogeneous Mesoporous Networks Consisting of Variable Connectivity and Pore-Size Correlation. Phys. Chem. Chem. Phys. 2002, 4, 2346–2355. [Google Scholar] [CrossRef]
- He, S.; Chen, G.; Xiao, H.; Shi, G.; Ruan, C.; Ma, Y.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Facile Preparation of N-Doped Activated Carbon Produced from Rice Husk for CO2 Capture. J. Colloid Interface Sci. 2021, 582, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Reichenauer, G. Structural Characterization of Aerogels. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Advances in Sol-Gel Derived Materials and Technologies; Springer: New York, NY, USA, 2011; pp. 449–498. ISBN 978-1-4419-7589-8. [Google Scholar]
- Reichenauer, G.; Heinemann, U.; Ebert, H.-P. Relationship between Pore Size and the Gas Pressure Dependence of the Gaseous Thermal Conductivity. Colloids Surf. Physicochem. Eng. Asp. 2007, 300, 204–210. [Google Scholar] [CrossRef]
- Shabanian, M.; Ardeshir, H.; Haji-Ali, S.; Moghanian, H.; Hajibeygi, M.; Faghihi, K.; Khonakdar, H.A.; Salimi, H. Efficient Poly(Methyl-Ether-Imide)/LDH Nanocomposite Derived from a Methyl Rich Bisphenol: From Synthesis to Properties. Appl. Clay Sci. 2016, 123, 285–291. [Google Scholar] [CrossRef]
- Coyote-Jiménez, J.C.; Zavala-Arce, R.E.; Jiménez-Núñez, M.L.; García-Rivas, J.L.; Rangel-Vázquez, N.A.; García-Gaitán, B. LDH-MgAl-NO3 Synthesis with the Intervention of Ethylenediamine Intended for the Removal of Fluoride Ions from Aqueous Solution. MRS Adv. 2021, 6, 985–988. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Leuteritz, A.; Kutlu, B.; der Landwehr, M.A.; Jehnichen, D.; Wagenknecht, U.; Heinrich, G. Preparation and Investigation of the Combustion Behavior of Polypropylene/Organomodified MgAl-LDH Micro-Nanocomposite. J. Alloys Compd. 2011, 509, 3497–3501. [Google Scholar] [CrossRef]
- Silva, T.S.; Fernandes, E.P.; Vithanage, M.; Meneghetti, S.M.P.; Meili, L. A Facile Synthesis of MgAl/Layered Double Hydroxides from Aluminum Wastes. Mater. Lett. 2022, 324, 132624. [Google Scholar] [CrossRef]
- Parvathy Rao, A.; Rao, A.V.; Pajonk, G.M. Hydrophobic and Physical Properties of the Ambient Pressure Dried Silica Aerogels with Sodium Silicate Precursor Using Various Surface Modification Agents. Appl. Surf. Sci. 2007, 253, 6032–6040. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Rao, A.V. Surface Chemical Modification of TEOS Based Silica Aerogels Synthesized by Two Step (Acid–Base) Sol–Gel Process. Appl. Surf. Sci. 2006, 252, 4289–4297. [Google Scholar] [CrossRef]
- Li, Z.; Huang, S.; Shi, L.; Li, Z.; Liu, Q.; Li, M. Reducing the Flammability of Hydrophobic Silica Aerogels by Doping with Hydroxides. J. Hazard. Mater. 2019, 373, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, S.; Koebel, M.M.; Malfait, W.J. Silica Aerogels with Tailored Chemical Functionality. Mater. Des. 2020, 193, 108833. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Rao, A.V.; Parale, V.G.; Kavale, M.S.; Wagh, P.B.; Ingale, S.V.; Gupta, S.C. Effect of Surface Composition and Roughness on the Apparent Surface Free Energy of Silica Aerogel Materials. Appl. Phys. Lett. 2011, 99, 104104. [Google Scholar] [CrossRef]
- Rao, A.V.; Kulkarni, M.M.; Amalnerkar, D.P.; Seth, T. Superhydrophobic Silica Aerogels Based on Methyltrimethoxysilane Precursor. J. Non-Cryst. Solids 2003, 330, 187–195. [Google Scholar] [CrossRef]
- He, S.; Huang, Y.; Chen, G.; Feng, M.; Dai, H.; Yuan, B.; Chen, X. Effect of Heat Treatment on Hydrophobic Silica Aerogel. J. Hazard. Mater. 2019, 362, 294–302. [Google Scholar] [CrossRef]
- Fernandes Júnior, A.J.S.; Sodré, W.C.; Soares, B.E.C.F.; Bezerra, C.W.B.; Rojas, A.; Perez-Carvajal, J.; Alcântara, A.C.S. In Situ Assembling of Layered Double Hydroxide to Magadiite Layered Silicate with Enhanced Photocatalytic and Recycling Performance. Appl. Surf. Sci. 2021, 569, 151007. [Google Scholar] [CrossRef]
- Hajibeygi, M.; Shabanian, M.; Omidi-Ghallemohamadi, M. Development of New Acid-Imide Modified Mg-Al/LDH Reinforced Semi-Crystalline Poly(Amide-Imide) Containing Naphthalene Ring; Study on Thermal Stability and Optical Properties. Appl. Clay Sci. 2017, 139, 9–19. [Google Scholar] [CrossRef]
- Lv, S.; Zhou, W.; Miao, H.; Shi, W. Preparation and Properties of Polymer/LDH Nanocomposite Used for UV Curing Coatings. Prog. Org. Coat. 2009, 65, 450–456. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Huang, S.; Liu, Q. Effects of Various Methylchlorosilanes on Physicochemical Properties of Ambient Pressure Dried Silica Aerogels. J. Nanopart. Res. 2019, 21, 234. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; Shi, L.; He, S.; Gong, L.; Li, C.; Zhang, H. Flammability and Oxidation Kinetics of Hydrophobic Silica Aerogels. J. Hazard. Mater. 2016, 320, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Rhen, C. Chemical Composition and Gross Calorific Value of the Above-Ground Biomass Components of Young Picea Abies. Scand. J. For. Res. 2004, 19, 72–81. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]














| Samples | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size * (nm) | Total Pore Volume (Vpore) (cm3/g) | Average Pore Diameter (Dpore) (nm) |
|---|---|---|---|---|---|
| SA | 903.7 | 2.8 | 12.2 | 8.5 | 37.6 |
| LDH/SA-5% | 864.3 | 2.7 | 12.4 | 8.1 | 37.7 |
| LDH/SA-10% | 814.5 | 2.7 | 13.1 | 7.5 | 36.7 |
| LDH/SA-20% | 730.7 | 2.9 | 15.6 | 7.3 | 39.9 |
| Tonset (°C) | Tpeak1 (°C) | Tpeak2 (°C) | Tpeak3 (°C) | |
|---|---|---|---|---|
| SA | 273.6 | 333.6 | ||
| LDH/SA-5% | 327.2 | 332.0 | 377.8 | 415.6 |
| LDH/SA-10% | 344.7 | 360.4 | 417.2 | 469.4 |
| LDH/SA-15% | 350.2 | 372.9 | 458.2 | 488.3 |
| LDH/SA-20% | 367.7 | 393.5 | 494.4 | 549.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Wang, Y.; Wang, X.; Liu, Q.; Li, M.; Shulga, Y.M.; Li, Z. In-Situ Synthesis of Layered Double Hydroxide/Silica Aerogel Composite and Its Thermal Safety Characteristics. Gels 2022, 8, 581. https://doi.org/10.3390/gels8090581
Sun M, Wang Y, Wang X, Liu Q, Li M, Shulga YM, Li Z. In-Situ Synthesis of Layered Double Hydroxide/Silica Aerogel Composite and Its Thermal Safety Characteristics. Gels. 2022; 8(9):581. https://doi.org/10.3390/gels8090581
Chicago/Turabian StyleSun, Mengtian, Yang Wang, Xiaowu Wang, Qiong Liu, Ming Li, Yury M. Shulga, and Zhi Li. 2022. "In-Situ Synthesis of Layered Double Hydroxide/Silica Aerogel Composite and Its Thermal Safety Characteristics" Gels 8, no. 9: 581. https://doi.org/10.3390/gels8090581