Imatinib Mesylate-Loaded Rosin/Cinnamon Oil-Based In Situ Forming Gel against Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rheological Characterization
2.2. Injectability and Mechanical Properties
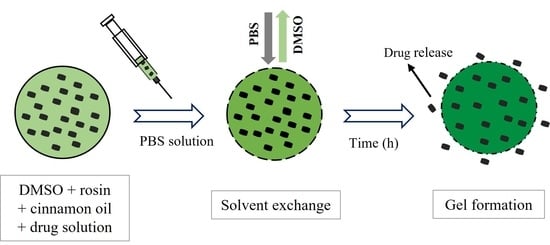
2.3. Gel Formation
2.4. Interfacial Phenomena
2.5. In Vitro Drug Release
2.5.1. Effect of the Rosin Concentration
2.5.2. Effect of the CO Content
2.6. Scanning Electron Microscopy (SEM)
2.7. Cytotoxicity
2.7.1. Cytotoxicity of Rosin
2.7.2. Cytotoxicity of ISG Formulations
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of ISG
4.3. Rheology and Viscosity Characterization
4.4. Injectability and Mechanical Properties
4.5. Gel Formation Study
4.6. Interfacial Phenomena
4.7. In Vitro Drug Release Studies
4.8. Scanning Electron Microscopy (SEM)
4.9. Cytotoxicity
4.10. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A. Colorectal cancer statistics. Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, F.; Eltarhoni, K.; Nisar, P.; Soloviev, M. Colorectal cancer diagnosis: The obstacles we face in determining a non-invasive test and current advances in biomarker detection. Cancers 2022, 14, 1889. [Google Scholar] [CrossRef] [PubMed]
- Maspes, A.; Pizzetti, F.; Rossetti, A.; Makvandi, P.; Sitia, G.; Rossi, F. Advances in bio-based polymers for colorectal cancer treatment: Hydrogels and nanoplatforms. Gels 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Ahnen, D.J. The American College of Gastroenterology Emily Couric Lecture—The adenoma-carcinoma sequence revisited: Has the era of genetic tailoring finally arrived? Am. J. Gastroenterol. 2011, 106, 190–198. [Google Scholar] [CrossRef]
- Shussman, N.; Wexner, S.D. Colorectal polyps and polyposis syndromes. Gastroenterol. Rep. 2014, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bhimani, N.; Wong, G.Y.M.; Molloy, C.; Dieng, M.; Hugh, T.J. Cost of colorectal cancer by treatment type from different health economic perspectives: A systematic review. Eur. J. Surg. Oncol. 2022, in press. [CrossRef]
- Yun, Q.; Wang, S.S.; Xu, S.; Yang, J.P.; Fan, J.; Yang, L.L.; Chen, Y.; Fu, S.Z.; Wu, J.B. Use of 5-Fluorouracil Loaded Micelles and Cisplatin in Thermosensitive Chitosan Hydrogel as an Efficient Therapy against Colorectal Peritoneal Carcinomatosis. Macromol. Biosci. 2017, 17, 1600262. [Google Scholar] [CrossRef]
- Hakim, L.; Alias, E.; Makpol, S.; Ngah, W.Z.; Morad, N.A.; Yusof, Y.A. Gelam honey and ginger potentiate the anti cancer effect of 5-FU against HCT 116 colorectal cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 4651–4657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, N.; Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.K.; Azab, S.S.; Youssef, S.S.; El-Sayed, A.M.; El-Demerdash, E.; Shouman, S. Modulation of imatinib cytotoxicity by selenite in HCT116 colorectal cancer cells. Basic Clin. Pharmacol. Toxicol. 2015, 116, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.A.; El-Sawy, H.S.; Abd-Allah, F.I.; Abdelghany, T.M.; El-Say, K.M. Maximizing the Therapeutic Efficacy of Imatinib Mesylate-Loaded Niosomes on Human Colon Adenocarcinoma Using Box-Behnken Design. J. Pharm. Sci. 2017, 106, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Benny, O.; Menon, L.G.; Ariel, G.; Goren, E.; Kim, S.K.; Stewman, C.; Black, P.M.; Carroll, R.S.; Machluf, M. Local delivery of poly lactic-co-glycolic acid microspheres containing imatinib mesylate inhibits intracranial xenograft glioma growth. Clin. Cancer Res. 2009, 15, 1222–1231. [Google Scholar] [CrossRef] [Green Version]
- Gursale, A.; Dighe, V.; Parekh, G. Simultaneous quantitative determination of cinnamaldehyde and methyl eugenol from stem bark of Cinnamomum zeylanicum blume using RP-HPLC. J. Chromatogr. Sci. 2010, 48, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Larasati, Y.A.; Meiyanto, E. Revealing the Potency of Cinnamon as an Anti-cancer and Chemopreventive Agent. Indones. J. Cancer Chemoprevention 2018, 9, 47–62. [Google Scholar] [CrossRef]
- Behrooz Alizadeh, B.; Falah, F.; Lavi Arab, F.; Vasiee, M.; Tabatabaee Yazdi, F. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid. Based Complement. Altern. Med. 2020, 2020, 5190603. [Google Scholar] [CrossRef]
- Elgendy, E.M.; Ibrahim, H.S.; Elmeherry, H.F.; Sedki, A.G.; Mekhemer, F.U. Chemical and biological comparative in vitro studies of cinnamon bark and lemon peel essential oils. Food Nutr. Sci. 2017, 8, 110–125. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Yang, H.; Lee, I.Y.; Yong, T.S.; Lee, S. Core/Sheath-Structured Composite Nanofibers Containing Cinnamon Oil: Their Antibacterial and Antifungal Properties and Acaricidal Effect against House Dust Mites. Polymers 2020, 12, 243. [Google Scholar] [CrossRef] [Green Version]
- Khaing, E.M.; Phaechamud, T.; Intaraphairot, T. Synergistic anticancer activity of cinnamon bark oil and imatinib mesylate combination on colorectal cancer cell lines. Key Eng. Mater. 2022, 914, 93–98. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Alternat. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef] [Green Version]
- Phaechamud, T.; Thurein, S.M.; Chantadee, T. Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel. Asian J. Pharm. Sci. 2018, 13, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Mahadlek, J.; Tuntarawongsa, S. Peppermint oil/doxycycline hyclate-loaded Eudragit RS in situ forming gel for periodontitis treatment. J. Pharm. Investig. 2017, 48, 451–464. [Google Scholar] [CrossRef]
- Mahadlek, J.; Rein, S.M.T.; Chinpaisal, C.; Phaechamud, T. Physical properties and bioactivity of clove oil-loaded solvent exchange-induced in situ forming gel. Mater. Today Proc. 2021, 47, 3509–3516. [Google Scholar] [CrossRef]
- Garala, K.; Joshi, P.; Shah, M.; Ramkishan, A.; Patel, J. Formulation and evaluation of periodontal in situ gel. Int. J. Pharm. Invest 2013, 3, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Gao, X.; Zhao, Y.; Xu, H.; Yang, X. The dual temperature/pH-sensitive multiphase behavior of poly (N-isopropylacrylamide-co-acrylic acid) microgels for potential application in in situ gelling system. Colloids Surf. B 2011, 84, 103–110. [Google Scholar] [CrossRef]
- Khattab, A.; Abouhussein, D.M.N.; Mohammad, F.E. Development of injectable tenoxicam in situ forming microparticles based on sesame oil and poly-DL-lactide: Characterization, efficacy and acute toxicity. J. Drug Deliv. Sci. Technol. 2019, 51, 682–694. [Google Scholar] [CrossRef]
- Ranch, K.M.; Maulvi, F.A.; Koli, A.R.; Desai, D.T.; Parikh, R.K.; Shah, D.O. Tailored doxycycline hyclate loaded in situ gel for the treatment of periodontitis: Optimization, in vitro characterization, and antimicrobial studies. AAPS PharmSciTech 2021, 22, 77. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; Han, B.; Zhao, N.; Mu, M.; Wang, C.; Du, Y.; Wang, Y.; Tong, A.; Liu, Y.; et al. Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur. J. Pharm. Sci. 2019, 128, 279–289. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, Y.; Jv, M. Synthesis, characterization and evaluation of tinidazole-loaded mPEG-PDLLA (10/90) in situ gel forming system for periodontitis treatment. Drug Deliv. 2016, 23, 2726–2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasongkla, N.; Nittayacharn, P.; Rotjanasitthikit, A.; Pungbangkadee, K.; Manaspon, C. Paclitaxel-loaded polymeric depots as injectable drug delivery system for cancer chemotherapy of hepatocellular carcinoma. Pharm. Dev. Technol. 2017, 22, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Hu, J.; Zhang, L.; Zhang, L.; Sun, Y.; Xie, Y.; Wu, S.; Liu, L.; Gao, Z. Doxorubicin-loaded zein in situ gel for interstitial chemotherapy of colorectal cancer. Acta Pharm. Sin. B 2012, 2, 610–614. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.W.; Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Jin, L.M.; Lee, B.; Kim, J.H.; Min, B.H.; Kim, M.S. Injectable intratumoral hydrogel as 5-fluorouracil drug depot. Biomaterials 2013, 34, 2748–2757. [Google Scholar] [CrossRef]
- Nittayacharn, P.; Nasongkla, N. Development of self-forming doxorubicin-loaded polymeric depots as an injectable drug delivery system for liver cancer chemotherapy. J. Mater. Sci. Mater. Med. 2017, 28, 101. [Google Scholar] [CrossRef]
- Lin, H.R.; Tseng, C.C.; Lin, Y.J.; Ling, M.H. A novel in-situ-gelling liquid suppository for site-targeting delivery of anti-colorectal cancer drugs. J. Biomater. Sci. Polym. Ed. 2012, 23, 807–822. [Google Scholar] [CrossRef]
- Satturwar, P.M.; Fulzele, S.V.; Dorle, A.K. Biodegradation and in vivo biocompatibility of rosin: A natural film forming polymer. AAPS PharmSciTech 2003, 4, 434–439. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid /chitosan composite film for food packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef]
- Santovito, E.; das Neves, J.; Greco, D.; D’Ascanio, V.; Sarmento, B.; Logrieco, A.F.; Avantaggiato, G. Antimicrobial properties of rosin acids-loaded nanoparticles against antibiotic-sensitive and antibiotic-resistant foodborne pathogens. Artif. Cells Nanomed. Biotechnol. 2018, 46, S414–S422. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Zhang, W.; Song, J.; Zhang, Y.; Jiang, X.; Wang, R. Anticancer effects of a novel class rosin-derivatives with different mechanisms. Bioorg. Med. Chem. Lett. 2013, 23, 3868–3872. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Liu, Y.; Wang, C.; Chu, F. Preparation and characterization of natural rosin stabilized nanoparticles via miniemulsion polymerization and their pressure-sensitive adhesive applications. Ind. Crops Prod. 2018, 124, 244–253. [Google Scholar] [CrossRef]
- Singh, V.; Joshi, S.; Malviya, T. Carboxymethyl cellulose-rosin gum hybrid nanoparticles: An efficient drug carrier. Int. J. Biol. Macromol. 2018, 112, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-M.; Lim, S.; Kim, G.-Y.; Kim, D.; Kim, D.-W.; Lee, H.-C.; Lee, K.-Y. Rosin microparticles as drug carriers: Influence of various solvents on the formation of particles and sustained-release of indomethacin. Biotechnol. Bioprocess Eng. 2004, 9, 476–481. [Google Scholar] [CrossRef]
- Khaing, E.M.; Intaraphairot, T.; Chuenbarn, T.; Chantadee, T.; Phaechamud, T. Natural resin-based solvent exchange induced in-situ forming gel for vancomycin HCl delivery to periodontal pocket. Mater. Today Proc. 2021, 47, 3585–3593. [Google Scholar] [CrossRef]
- Khaing, E.M.; Mahadlek, J.; Okonogi, S.; Phaechamud, T. Lime peel oil-incorporated rosin-based antimicrobial in situ forming gel. Gels 2022, 8, 169. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-based antisolvent-induced in situ forming matrix for crevicular pocket delivery of vancomycin hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef]
- Xuan, J.J.; Yan, Y.D.; Oh, D.H.; Choi, Y.K.; Yong, C.S.; Choi, H.G. Development of thermo-sensitive injectable hydrogel with sustained release of doxorubicin: Rheological characterization and in vivo evaluation in rats. Drug Deliv. 2011, 18, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Xuan, J.J.; Balakrishnan, P.; Oh, D.H.; Yeo, W.H.; Park, S.M.; Yong, C.S.; Choi, H.G. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int. J. Pharm. 2010, 395, 317–323. [Google Scholar] [CrossRef]
- Nasra, M.M.; Khiri, H.M.; Hazzah, H.A.; Abdallah, O.Y. Formulation, in-vitro characterization and clinical evaluation of curcumin in-situ gel for treatment of periodontitis. Drug Deliv. 2017, 24, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Altuntas, E.; Yener, G. Formulation and evaluation of thermoreversible in situ nasal gels containing mometasone furoate for allergic rhinitis. AAPS PharmSciTech 2017, 18, 2673–2682. [Google Scholar] [CrossRef]
- Agossa, K.; Lizambard, M.; Rongthong, T.; Delcourt-Debruyne, E.; Siepmann, J.; Siepmann, F. Physical key properties of antibiotic-free, PLGA/HPMC-based in-situ forming implants for local periodontitis treatment. Int. J. Pharm. 2017, 521, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Thurein, S.M.; Lertsuphotvanit, N.; Phaechamud, T. Physicochemical properties of beta-cyclodextrin solutions and precipitates prepared from injectable vehicles. Asian J. Pharm. Sci. 2018, 13, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Rein, S.M.T.; Lwin, W.W.; Tuntarawongsa, S.; Phaechamud, T. Meloxicam-loaded solvent exchange-induced in situ forming beta-cyclodextrin gel and microparticle for periodontal pocket delivery. Mater. Sci. Eng. C 2020, 117, 111275. [Google Scholar] [CrossRef] [PubMed]
- Rungseevijitprapa, W.; Bodmeier, R. Injectability of biodegradable in situ forming microparticle systems (ISM). Eur. J. Pharm. Sci. 2009, 36, 524–531. [Google Scholar] [CrossRef]
- Xu, C.-A.; Qu, Z.; Lu, M.; Meng, H.; Zhan, Y.; Chen, B.; Wu, K.; Shi, J. Effect of rosin on the antibacterial activity against S.aureus and adhesion properties of UV-curable polyurethane/polysiloxane pressure-sensitive adhesive. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126146. [Google Scholar] [CrossRef]
- Chuenbarn, T.; Sirirak, J.; Tuntarawongsa, S.; Okonogi, S.; Phaechamud, T. Design and Comparative Evaluation of Vancomycin HCl-Loaded Rosin-Based in Situ Forming Gel and Microparticles. Gels 2022, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Zahir-Jouzdani, F.; Wolf, J.D.; Atyabi, F.; Bernkop-Schnurch, A. In situ gelling and mucoadhesive polymers: Why do they need each other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef]
- De France, K.J.; Cranston, E.D.; Hoare, T. Mechanically reinforced injectable hydrogels. ACS Appl. Polym. Mater. 2020, 2, 1016–1030. [Google Scholar] [CrossRef]
- Bloch, Y.; Mendlovic, S.; Strupinsky, S.; Altshuler, A. Injections of depot antipsychotic medications in patients suffering from schizophrenia. J. Clin. Psychiatry 2001, 62, 855–859. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Eissa, R.G.; El-Megrab, N.A.; El-Nahas, H.M. Morphological characterization of optimized risperidone-loaded in-situ gel forming implants with pharmacokinetic and behavioral assessments in rats. J. Drug Deliv. Sci. Technol. 2021, 61, 102195. [Google Scholar] [CrossRef]
- Solorio, L.; Olear, A.M.; Zhou, H.; Beiswenger, A.C.; Exner, A.A. Effect of cargo properties on in situ forming implant behavior determined by noninvasive ultrasound imaging. Drug Deliv. Transl. Res. 2012, 2, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, T.A.; Ibrahim, H.M.; Ibrahim, F.; Samy, A.M.; Kaseem, A.; Nutan, M.T.; Hussain, M.D. Development of biodegradable in situ implant and microparticle injectable formulations for sustained delivery of haloperidol. J. Pharm. Sci. 2012, 101, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, Z.; Mustafa, W.W.; Abdelkader, H.; Moharram, H.; Sabry, A.M.; Alany, R.G. Hybrid thermosensitive-mucoadhesive in situ forming gels for enhanced corneal wound healing effect of L-carnosine. Drug Deliv. 2022, 29, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Phaechamud, T. Saturated fatty acid-based in situ forming matrices for localized antimicrobial delivery. Pharmaceutics 2020, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Jantadee, T.; Mahadlek, J.; Charoensuksai, P.; Pichayakorn, W. Characterization of Antimicrobial Agent Loaded Eudragit RS Solvent Exchange-Induced in Situ Forming Gels for Periodontitis Treatment. AAPS PharmSciTech 2017, 18, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Luo, X.; Zhang, Z.; Li, H.; Tian, J.; Yu, Z. Study of an injectable in situ forming gel for sustained-release of Ivermectin in vitro and in vivo. Int. J. Biol. Macromol. 2016, 85, 271–276. [Google Scholar] [CrossRef]
- Yoo, J.; Won, Y.Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, D.; Tian, Y.; Li, H.; Gong, T.; Luo, J.; Ruan, J.; Gong, T.; Zhang, Z. Comparison of three in-situ gels composed of different oil types. Int. J. Pharm. 2020, 587, 119707. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Kruszka, A.; Siepmann, F.; Siepmann, J. In-situ forming PLGA implants: How additives affect swelling and drug release. J. Drug Deliv. Sci. Technol. 2019, 53, 101180. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Haffez, H.; Osman, S.; Ebrahim, H.Y.; Hassan, Z.A. Growth Inhibition and Apoptotic Effect of Pine Extract and Abietic Acid on MCF-7 Breast Cancer Cells via Alteration of Multiple Gene Expressions Using In Vitro Approach. Molecules 2022, 27, 293. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.F. Imatinib mesylate. Recent Results Cancer Res. 2018, 212, 1–27. [Google Scholar] [PubMed]
- Komeda, S.; Uemura, M.; Yoneyama, H.; Harusawa, S.; Hiramoto, K. In vitro cytotoxicity and in vivo antitumor efficacy of tetrazolato-bridged dinuclear platinum(II) complexes with a bulky substituent at tetrazole C5. Inorganics 2019, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Solano, L.N.; Nelson, G.L.; Ronayne, C.T.; Jonnalagadda, S.; Jonnalagadda, S.K.; Kottke, K.; Chitren, R.; Johnson, J.L.; Pandey, M.K.; Jonnalagadda, S.C.; et al. Synthesis, in vitro, and in vivo evaluation of novel N-phenylindazolyl diarylureas as potential anti-cancer agents. Sci. Rep. 2020, 10, 17969. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, A.; Zhao, X.; Liu, X.; Wang, D.; Sun, F.; Li, Y. Design of a long-term antipsychotic in situ forming implant and its release control method and mechanism. Int. J. Pharm. 2012, 427, 284–292. [Google Scholar] [CrossRef]
- Madsen, B.K.; Hilscher, M.; Zetner, D.; Rosenberg, J. Adverse reactions of dimethyl sulfoxide in humans: A systematic review. F1000Research 2018, 7, 1746. [Google Scholar] [CrossRef]
- Colaco, M.A.; Evans, R.J. Current recommendations for bladder instillation therapy in the treatment of interstitial cystitis/bladder pain syndrome. Curr. Urol. Rep. 2013, 14, 442–447. [Google Scholar] [CrossRef]
- Do, M.P.; Neut, C.; Delcourt, E.; Seixas Certo, T.; Siepmann, J.; Siepmann, F. In situ forming implants for periodontitis treatment with improved adhesive properties. Eur. J. Pharm. Biopharm. 2014, 88, 342–350. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Chantadee, T.; Sawangsri, P.; Santimaleeworagun, W.; Phaechamud, T. Vancomycin hydrochloride-loaded stearic acid/lauric acid in situ forming matrix for antimicrobial inhibition in patients with joint infection after total knee arthroplasty. Mater. Sci. Eng. C 2020, 115, 110761. [Google Scholar] [CrossRef]










| Formula | Work of Injectability (N.mm) | Hardness | Adhesion | |
|---|---|---|---|---|
| 21-Guage | 24-Guage | (n) | (n) | |
| IM-R35 | 4.90 ± 0.37 | 13.21 ± 1.57 | 0.275 ± 0.03 | 0.028 ± 0.02 |
| IM-R45 | 7.39 ± 0.50 | 24.91 ± 1.34 | 0.433 ± 0.03 | 0.030 ± 0.01 |
| IM-R55 | 16.16 ± 0.98 | 84.08 ± 8.31 | 0.580 ± 0.02 | 0.040 ± 0.00 |
| IM-RC2.5 | 18.71 ± 1.35 | 104.1 ± 5.83 | 0.437 ± 0.03 | 0.037 ± 0.01 |
| IM-RC5 | 23.33 ± 3.04 | 125.0 ± 11.3 | 0.204 ± 0.03 | 0.042 ± 0.01 |
| IM-RC10 | 27.10 ± 2.82 | 154.6 ± 10.0 | 0.116 ± 0.02 | 0.128 ± 0.00 |
| Formula | Zero Order | First Order | Higuchi’s | Korsmeyer–Peppas | ||
|---|---|---|---|---|---|---|
| r2 | r2 | r2 | r2 | n | Release Mechanism | |
| IM-R35 | 0.799 | 0.814 | 0.938 | 1.000 | 0.239 | Fickian diffusion |
| IM-R45 | 0.811 | 0.826 | 0.945 | 1.000 | 0.251 | Fickian diffusion |
| IM-R55 | 0.840 | 0.854 | 0.961 | 0.988 | 0.325 | Fickian diffusion |
| IM-RC2.5 | 0.820 | 0.846 | 0.941 | 0.956 | 0.379 | Fickian diffusion |
| IM-RC5 | 0.784 | 0.833 | 0.908 | 0.921 | 0.400 | Fickian diffusion |
| IM-RC10 | 0.895 | 0.942 | 0.974 | 0.975 | 0.488 | non-Fickian diffusion |
| Formula | IM | Rosin | CO | DMSO |
|---|---|---|---|---|
| (% w/w) | (% w/w) | (% w/w) | (% w/w) | |
| IM-R35 | 1 | 35 | - | 64 |
| IM-R45 | 1 | 45 | - | 54 |
| IM-R55 | 1 | 55 | - | 44 |
| IM-RC2.5 | 1 | 55 | 2.5 | 41.5 |
| IM-RC5 | 1 | 55 | 5 | 39 |
| IM-RC10 | 1 | 55 | 10 | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaing, E.M.; Intaraphairot, T.; Mahadlek, J.; Okonogi, S.; Pichayakorn, W.; Phaechamud, T. Imatinib Mesylate-Loaded Rosin/Cinnamon Oil-Based In Situ Forming Gel against Colorectal Cancer Cells. Gels 2022, 8, 526. https://doi.org/10.3390/gels8090526
Khaing EM, Intaraphairot T, Mahadlek J, Okonogi S, Pichayakorn W, Phaechamud T. Imatinib Mesylate-Loaded Rosin/Cinnamon Oil-Based In Situ Forming Gel against Colorectal Cancer Cells. Gels. 2022; 8(9):526. https://doi.org/10.3390/gels8090526
Chicago/Turabian StyleKhaing, Ei Mon, Torsak Intaraphairot, Jongjan Mahadlek, Siriporn Okonogi, Wiwat Pichayakorn, and Thawatchai Phaechamud. 2022. "Imatinib Mesylate-Loaded Rosin/Cinnamon Oil-Based In Situ Forming Gel against Colorectal Cancer Cells" Gels 8, no. 9: 526. https://doi.org/10.3390/gels8090526