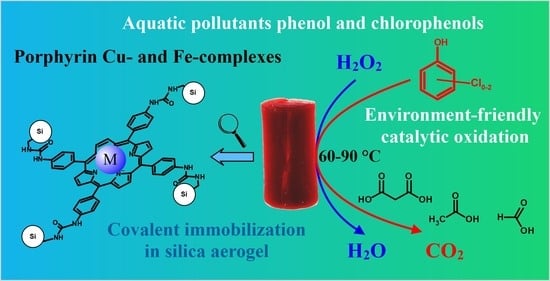
Environment-Friendly Catalytic Mineralization of Phenol and Chlorophenols with Cu- and Fe- Tetrakis(4-aminophenyl)-porphyrin—Silica Hybrid Aerogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of the Heterogeneous Catalysts
2.2. Characterization of the Catalysts




2.3. Catalytic Activity
2.3.1. Phenol Oxidation
2.3.2. Oxidation of 3-Chloro- and 2,4-Dichlorophenol
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Porphyrin Complexes
4.3. Synthesis of Heterogeneous Catalysts
4.4. Characterization of the Catalysts
4.5. Study of Catalytic Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahlborg, U.G.; Thunberg, T.M.; Spencer, H.C. Chlorinated Phenols: Occurrence, Toxicity, Metabolism, And Environmental Impact. CRC Crit. Rev. Toxicol. 1980, 7, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Decision No 2455/2001/EC of the European Parliament and of the Council of 20 November 2001 Establishing the List of Priority Substances in the Field of Water Policy and Amending Directive 2000/60/EC. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:331:0001:0005:EN:PDF (accessed on 2 February 2022).
- Goel, M.; Chovelon, J.-M.; Ferronato, C.; Bayard, R.; Sreekrishnan, T.R. The remediation of wastewater containing 4-chlorophenol using integrated photocatalytic and biological treatment. J. Photochem. Photobiol. B Biol. 2010, 98, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sahinkaya, E.; Dilek, F.B. Biodegradation of 4-CP and 2,4-DCP mixture in a rotating biological contactor (RBC). Biochem. Eng. J. 2006, 31, 141–147. [Google Scholar] [CrossRef]
- Field, J.A.; Sierra-Alvarez, R. Microbial degradation of chlorinated phenols. Rev. Environ. Sci. Bio/Technol. 2008, 7, 211–241. [Google Scholar] [CrossRef]
- Barik, A.J.; Gogate, P.R. Hybrid treatment strategies for 2,4,6-trichlorophenol degradation based on combination of hydrodynamic cavitation and AOPs. Ultrason. Sonochem. 2018, 40, 383–394. [Google Scholar] [CrossRef]
- Tai, C.; Jiang, G. Dechlorination and destruction of 2,4,6-trichlorophenol and pentachlorophenol using hydrogen peroxide as the oxidant catalyzed by molybdate ions under basic condition. Chemosphere 2005, 59, 321–326. [Google Scholar] [CrossRef]
- Xiong, J.; Hang, C.; Gao, J.; Guo, Y.; Gu, C. A novel biomimetic catalyst templated by montmorillonite clay for degradation of 2,4,6-trichlorophenol. Chem. Eng. J. 2014, 254, 276–282. [Google Scholar] [CrossRef]
- Wang, L.; Kong, D.; Ji, Y.; Lu, J.; Yin, X.; Zhou, Q. Formation of halogenated disinfection byproducts during the degradation of chlorophenols by peroxymonosulfate oxidation in the presence of bromide. Chem. Eng. J. 2018, 343, 235–243. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.-D.; Liu, L.; Liu, Y.; Zhang, H.-W.; Han, X. Efficient oxidation of phenol by persulfate using manganite as a catalyst. J. Mol. Catal. A Chem. 2016, 411, 264–271. [Google Scholar] [CrossRef]
- Bossmann, S.H.; Oliveros, E.; Göb, S.; Siegwart, S.; Dahlen, E.P.; Payawan, J.L.; Straub, M.; Wörner, A.M.; Braun, A.M. New Evidence against Hydroxyl Radicals as Reactive Intermediates in the Thermal and Photochemically Enhanced Fenton Reactions. J. Phys. Chem. A 1998, 102, 5542–5550. [Google Scholar] [CrossRef]
- Barbusinski, K. Fenton Reaction—Controversy Concerning The Chemistry. Ecol. Chem. Eng. S 2009, 16, 347–358. [Google Scholar]
- Ross, J.R.H. Heterogeneous Catalysis: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-444-53363-0. [Google Scholar]
- Davis, M.E.; Davis, R.J. Fundamentals of Chemical Reaction Engineering, International ed.; McGraw-Hill Chemical Engineering Series; McGraw-Hill: Boston, MA, USA, 2003; ISBN 978-0-07-245007-1. [Google Scholar]
- Jiang, S.; Zhang, H.; Yan, Y. Catalytic wet peroxide oxidation of phenol wastewater over a novel Cu–ZSM-5 membrane catalyst. Catal. Commun. 2015, 71, 28–31. [Google Scholar] [CrossRef]
- Shukla, P.; Wang, S.; Singh, K.; Ang, H.; Tade, M. Cobalt exchanged zeolites for heterogeneous catalytic oxidation of phenol in the presence of peroxymonosulphate. Appl. Catal. B Environ. 2010, 99, 163–169. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, S.; Zhang, H.; Zhang, X. Preparation of novel Fe-ZSM-5 zeolite membrane catalysts for catalytic wet peroxide oxidation of phenol in a membrane reactor. Chem. Eng. J. 2015, 259, 243–251. [Google Scholar] [CrossRef]
- El-Remaily, M.A.E.A.A.A.; Elhady, O. Cobalt(III)–porphyrin complex (CoTCPP) as an efficient and recyclable homogeneous catalyst for the synthesis of tryptanthrin in aqueous media. Tetrahedron Lett. 2016, 57, 435–437. [Google Scholar] [CrossRef]
- Miyamoto, T.; Zhu, Q.; Igrashi, M.; Kodama, R.; Maeno, S.; Fukushima, M. Catalytic oxidation of tetrabromobisphenol A by iron(III)-tetrakis(p-sulfonatephenyl)porphyrin catalyst supported on cyclodextrin polymers with potassium monopersulfate. J. Mol. Catal. B Enzym. 2015, 119, 64–70. [Google Scholar] [CrossRef]
- Najafian, A.; Rabbani, M.; Rahimi, R.; Deilamkamar, M.; Maleki, A. Synthesis and characterization of copper porphyrin into SBA-16 through “ship in a bottle” method: A catalyst for photo oxidation reaction under visible light. Solid State Sci. 2015, 46, 7–13. [Google Scholar] [CrossRef]
- Fukushima, M.; Tatsumi, K. Complex formation of water-soluble iron(III)-porphyrin with humic acids and their effects on the catalytic oxidation of pentachlorophenol. J. Mol. Catal. A Chem. 2006, 245, 178–184. [Google Scholar] [CrossRef]
- Nappa, M.J.; Tolman, C.A. Steric and electronic control of iron porphyrin catalyzed hydrocarbon oxidations. Inorg. Chem. 1985, 24, 4711–4719. [Google Scholar] [CrossRef]
- Rubin, M.; Lampert, C.M. Transparent silica aerogels for window insulation. Sol. Energy Mater. 1983, 7, 393–400. [Google Scholar] [CrossRef]
- Tabata, M.; Adachi, I.; Hatakeyama, Y.; Kawai, H.; Morita, T.; Sumiyoshi, T. Large-area silica aerogel for use as Cherenkov radiators with high refractive index, developed by supercritical carbon dioxide drying. J. Supercrit. Fluids 2016, 110, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Bereczki, H.F.; Daróczi, L.; Fábián, I.; Lázár, I. Sol-gel synthesis, characterization and catalytic activity of silica aerogels functionalized with copper(II) complexes of cyclen and cyclam. Microporous Mesoporous Mater. 2016, 234, 392–400. [Google Scholar] [CrossRef]
- Lázár, I.; Kalmár, J.; Peter, A.; Szilágyi, A.; Győri, E.; Ditrói, T.; Fábián, I. Photocatalytic performance of highly amorphous titania–silica aerogels with mesopores: The adverse effect of the in situ adsorption of some organic substrates during photodegradation. Appl. Surf. Sci. 2015, 356, 521–531. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.; Bang, Y.; Han, S.J.; Park, S.; Song, J.H.; Song, I.K. Hydrogen production by tri-reforming of methane over nickel–alumina aerogel catalyst. J. Mol. Catal. A Chem. 2015, 410, 74–80. [Google Scholar] [CrossRef]
- Amiri, T.Y.; Moghaddas, J. Cogeled copper-silica aerogel as a catalyst in hydrogen production from methanol steam reforming. Int. J. Hydrog. Energy 2015, 40, 1472–1480. [Google Scholar] [CrossRef]
- Hu, E.; Wu, X.; Shang, S.; Tao, X.; Jiang, S.; Gan, L. Catalytic ozonation of simulated textile dyeing wastewater using mesoporous carbon aerogel supported copper oxide catalyst. J. Clean. Prod. 2015, 112, 4710–4718. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.-B.; Li, J.-L.; Zhang, J.-J.; Sui, X.; Zhang, L.-M. Hybrid of carbon-supported Pt nanoparticles and three dimensional graphene aerogel as high stable electrocatalyst for methanol electrooxidation. Electrochim. Acta 2016, 189, 175–183. [Google Scholar] [CrossRef]
- Liu, M.; Peng, C.; Yang, W.; Guo, J.; Zheng, Y.; Chen, P.; Huang, T.; Xu, J. Pd nanoparticles supported on three-dimensional graphene aerogels as highly efficient catalysts for methanol electrooxidation. Electrochim. Acta 2015, 178, 838–846. [Google Scholar] [CrossRef]
- Balantseva, E.; Miletto, I.; Coluccia, S.; Berlier, G. Immobilisation of Zinc porphyrins on mesoporous SBA-15: Effect of bulky substituents on the surface interaction. Microporous Mesoporous Mater. 2014, 193, 103–110. [Google Scholar] [CrossRef]
- Silva, M.; Azenha, M.; Pereira, M.; Burrows, H.; Sarakha, M.; Forano, C.; Ribeiro, M.F.; Fernandes, A. Immobilization of halogenated porphyrins and their copper complexes in MCM-41: Environmentally friendly photocatalysts for the degradation of pesticides. Appl. Catal. B Environ. 2010, 100, 1–9. [Google Scholar] [CrossRef]
- McDonald, A.R.; Franssen, N.; van Klink, G.P.; van Koten, G. ‘Click’ silica immobilisation of metallo-porphyrin complexes and their application in epoxidation catalysis. J. Organomet. Chem. 2009, 694, 2153–2162. [Google Scholar] [CrossRef]
- Ribeiro, S.; Serra, A.; Gonsalves, A.R. Covalently immobilized porphyrins as photooxidation catalysts. Tetrahedron 2007, 63, 7885–7891. [Google Scholar] [CrossRef] [Green Version]
- García-Sánchez, M.; de la Luz, V.; Estrada-Rico, M.; Murillo-Martínez, M.; Coahuila-Hernández, M.; Sosa-Fonseca, R.; Tello-Solís, S.; Rojas, F.; Campero, A. Fluorescent porphyrins covalently bound to silica xerogel matrices. J. Non-Cryst. Solids 2009, 355, 120–125. [Google Scholar] [CrossRef]
- Lente, G.; Fábián, I. Kinetics and mechanism of the oxidation of water soluble porphyrin FeIIITPPS with hydrogen peroxide and the peroxomonosulfate ion. Dalton Trans. 2007, 4268–4275. [Google Scholar] [CrossRef]
- Lázár, I.; Fábián, I. A Continuous Extraction and Pumpless Supercritical CO2 Drying System for Laboratory-Scale Aerogel Production. Gels 2016, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Lian, W.; Sun, Y.; Wang, B.; Shan, N.; Shi, T. Synthesis and properties of 5,10,15,20-tetra[4-(3,5-dioctoxybenzamidephenyl] porphyrin and its metal complexes. J. Serb. Chem. Soc. 2012, 77, 335–348. [Google Scholar] [CrossRef]
- Kozlowski, P.M.; Jarzȩcki, A.A.; Pulay, P.; Li, X.-Y.; Zgierski, M.Z. Vibrational Assignment and Definite Harmonic Force Field for Porphine. 2. Comparison with Nonresonance Raman Data. J. Phys. Chem. 1996, 100, 13985–13992. [Google Scholar] [CrossRef]
- Pop, S.-F.; Ion, R.-M.; Corobea, M.C.; Raditoiu, V. Spectral and Thermal Investigations of Porphyrin and Phthalocyanine Nanomaterials. J. Optoelectron. Adv. Mater. 2011, 13, 906–911. [Google Scholar]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Serra, A.C.; Gonsalves, A.R. Covalently immobilized porphyrins on silica modified structures as photooxidation catalysts. J. Mol. Catal. A Chem. 2010, 326, 121–127. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Pliego, G.; Casas, J.A.; Rodriguez, J.J.; Garcia, M.M. Chlorinated Byproducts from the Fenton-like Oxidation of Polychlorinated Phenols. Ind. Eng. Chem. Res. 2012, 51, 13092–13099. [Google Scholar] [CrossRef]
- Hagen, J. Industrial Catalysis: A Practical Approach, 3rd Completely Revised and Enlarged ed.; Wiley-VCH: Weinheim, Germany, 2015; ISBN 978-3-527-33165-9. [Google Scholar]
- Toxicological Profile for Chlorophenols Draft for Public Comment. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp107.pdf (accessed on 4 February 2022).
- Zhao, X.; Liu, X.; Yu, M.; Wang, C.; Li, J. The highly efficient and stable Cu, Co, Zn-porphyrin–TiO2 photocatalysts with heterojunction by using fashioned one-step method. Dye. Pigment. 2016, 136, 648–656. [Google Scholar] [CrossRef]











| Sample\Element | C (%) | H (%) | N (%) |
|---|---|---|---|
| “blank” silica aerogel | 0.75 | 1.41 | 0.36 |
| CuPA | 1.93 | 1.70 | 0.51 |
| FePA | 3.21 | 1.80 | 0.69 |
| CuPA | FePA | |
|---|---|---|
| SBET (m2/g) | 980 | 1019 |
| d (nm) | 12.8 | 11.4 |
| Vtotal (cm3/g) | 3.617 | 3.9185 |
| Vmacro (cm3/g) | 0.3702 | 0.417 |
| Vmeso (cm3/g) | 3.2218 | 3.4975 |
| Vmicro (cm3/g) | 0.025 | 0.004 |
| Sample | Theoretical (w/w %) | Measured (w/w %) |
|---|---|---|
| CuPA | 0.068 | 0.072 ± 0.001 |
| FePA | 0.084 | 0.110 ± 0.001 |
| Sample | TOF (s−1) | Regression Coefficient |
|---|---|---|
| CuPA | 1.144 × 10−1 | 0.9816 |
| FePA | 4.05 × 10−2 | 0.9998 |
| 3-Chlorophenol | 2,4-Dichlorophenol | |||
|---|---|---|---|---|
| TOF (s−1) | Regression Coefficient | TOF (s−1) | Regression Coefficient | |
| CuPA | 5.0 × 10−2 | 0.9604 | 1.5 × 10−3 | 0.9884 |
| FePA | 5.0 × 10−2 | 0.9996 | 2.2 × 10−2 | 0.995 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Győri, E.; Kecskeméti, Á.; Fábián, I.; Szarka, M.; Lázár, I. Environment-Friendly Catalytic Mineralization of Phenol and Chlorophenols with Cu- and Fe- Tetrakis(4-aminophenyl)-porphyrin—Silica Hybrid Aerogels. Gels 2022, 8, 202. https://doi.org/10.3390/gels8040202
Győri E, Kecskeméti Á, Fábián I, Szarka M, Lázár I. Environment-Friendly Catalytic Mineralization of Phenol and Chlorophenols with Cu- and Fe- Tetrakis(4-aminophenyl)-porphyrin—Silica Hybrid Aerogels. Gels. 2022; 8(4):202. https://doi.org/10.3390/gels8040202
Chicago/Turabian StyleGyőri, Enikő, Ádám Kecskeméti, István Fábián, Máté Szarka, and István Lázár. 2022. "Environment-Friendly Catalytic Mineralization of Phenol and Chlorophenols with Cu- and Fe- Tetrakis(4-aminophenyl)-porphyrin—Silica Hybrid Aerogels" Gels 8, no. 4: 202. https://doi.org/10.3390/gels8040202