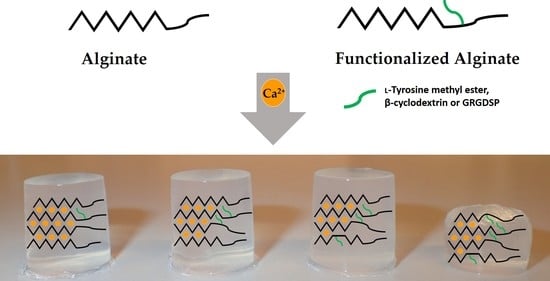
Mechanical Properties of Ca-Saturated Hydrogels with Functionalized Alginate
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization of the Functionalized Alginates
2.2. Ca-Saturated Alginate Hydrogels of Functionalized Alginates
2.3. Mechanical Properties of Mixed Ca-Gels of Oxidized Alginate and Stipe Alginate
2.4. Mechanical Properties of Mixed Ca-Saturated Hydrogels with Functionalized Alginate
2.5. Stability of Mixed Hydrogels and Leakage of Material upon Saline Exposure
3. Discussion
3.1. Mechanical Properties
3.2. Syneresis
3.3. Stability and Leakage
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Periodate Oxidation and Preparation of Functionalized Alginates
5.3. Preparation of Calcium–Alginate Hydrogels
5.4. Syneresis and Mechanical Properties
5.5. Rheological Characterisation of Unsaturated Gels
5.6. Stability
5.7. Analysis of the Leaked Alginate
5.8. NMR
5.9. SEC-MALS
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lutolf, M.P. Biomaterials: Spotlight on hydrogels. Nat. Mater. 2009, 8, 451–453. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Moe, S.T.; Skjåk-Bræk, G.; Smidsrød, O. Alginates. In Food Polysaccharides and Their Applications; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 289–334. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Rokstad, A.M.; Brekke, O.-L.; Steinkjer, B.; Ryan, L.; Kolláriková, G.; Strand, B.L.; Skjåk-Bræk, G.; Lacík, I.; Espevik, T.; Mollnes, T.E. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 2011, 7, 2566–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smidsrød, O. Molecular basis for some physical properties of alginates in the gel state. Faraday Discuss. Chem. Soc. 1974, 57, 263–274. [Google Scholar] [CrossRef]
- Smidsrød, O.; Haug, A. Dependence upon Uronic Acid Composition of Some Ion-Exchange Properties of Alginates. Acta Chem. Scand. 1968, 22, 1989–1997. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrød, O. Selectivity of Some Anionic Polymers for Divalent Metal Ions. Acta Chem. Scand. 1970, 24, 843–854. [Google Scholar] [CrossRef]
- Mørch, Y.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Donati, I.; Holtan, S.; Mørch, Y.A.; Borgogna, M.; Dentini, M.; Skjåk-Bræk, G. New hypothesis on the role of alternating sequences in calcium-alginate gels. Biomacromolecules 2005, 6, 1031–1040. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Holtan, S.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Mechanical Properties of C-5 Epimerized Alginates. Biomacromolecules 2008, 9, 2360–2368. [Google Scholar] [CrossRef]
- Donati, I.; Mørch, Y.A.; Strand, B.L.; Skjåk-Bræk, G.; Paoletti, S. Effect of Elongation of Alternating Sequences on Swelling Behavior and Large Deformation Properties of Natural Alginate Gels. J. Phys. Chem. B 2009, 113, 12916–12922. [Google Scholar] [CrossRef] [PubMed]
- Aarstad, O.; Strand, B.L.; Klepp-Andersen, L.M.; Skjåk-Bræk, G. Analysis of G-Block Distributions and Their Impact on Gel Properties of in Vitro Epimerized Mannuronan. Biomacromolecules 2013, 14, 3409–3416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haug, A. Composition and Properties of Alginates. Nor. Inst. Seaweed Res. 1964, 30. [Google Scholar]
- Gimmestad, M.; Sletta, H.; Ertesvag, H.; Bakkevig, K.; Jain, S.; Suh, S.; Skjåk-Bræk, G.; Ellingsen, T.E.; Ohman, D.E.; Valla, S. The Pseudomonas fluorescens AlgG protein, but not its mannuronan C-5-epimerase activity, is needed for alginate polymer formation. J. Bacteriol. 2003, 185, 3515–3523. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Molecular Engineering as an Approach to Design New Functional Properties of Alginate. Biomacromolecules 2007, 8, 2809–2814. [Google Scholar] [CrossRef]
- Fonseca, K.B.; Bidarra, S.J.; Oliveira, M.J.; Granja, P.L.; Barrias, C.C. Molecularly designed alginate hydrogels susceptible to local proteolysis as three-dimensional cellular microenvironments. Acta Biomater. 2011, 7, 1674–1682. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Sandvig, I.; Karstensen, K.; Rokstad, A.M.; Aachmann, F.L.; Formo, K.; Sandvig, A.; Skjåk-Bræk, G.; Strand, B.L. RGD-peptide modified alginate by a chemoenzymatic strategy for tissue engineering applications. J. Biomed. Mater. Res. Part A 2015, 103, 896–906. [Google Scholar] [CrossRef]
- Dalheim, M.Ø.; Vanacker, J.; Najmi, M.A.; Aachmann, F.L.; Strand, B.L.; Christensen, B.E. Efficient functionalization of alginate biomaterials. Biomaterials 2016, 80, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Carré, M.-C.; Delestre, C.; Hubert, P.; Dellacherie, E. Covalent coupling of a short polyether on sodium alginate: Synthesis and characterization of the resulting amphiphilic derivative. Carbohydr. Polym. 1991, 16, 367–379. [Google Scholar] [CrossRef]
- Andresen, I.-L.; Painter, T.; Smidsrød, O. Concerning the effect of periodate oxidation upon the intrinsic viscosity of alginate. Carbohydr. Res. 1977, 59, 563–566. [Google Scholar] [CrossRef]
- Kang, H.-A.; Jeon, G.-J.; Lee, M.-Y.; Yang, J.-W. Effectiveness test of alginate-derived polymeric surfactants. J. Chem. Technol. Biotechnol. 2002, 77, 205–210. [Google Scholar] [CrossRef]
- Dryhurst, G. Periodate Oxidation of Diol and Other Functional Groups: Analytical and Structural Applications; Pergamon Press: Oxford, UK, 1970. [Google Scholar]
- Lee, K.Y.; Bouhadir, K.H.; Mooney, D.J. Evaluation of chain stiffness of partially oxidized polyguluronate. Biomacromolecules 2002, 3, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Smidsrød, O.; Painter, T. Effect of periodate oxidation upon the stiffness of the alginate molecule in solution. Carbohydr. Res. 1973, 26, 125–132. [Google Scholar] [CrossRef]
- Vold, I.M.; Kristiansen, K.A.; Christensen, B.E. A study of the chain stiffness and extension of alginates, in vitro epimerized alginates, and periodate-oxidized alginates using size-exclusion chromatography combined with light scattering and viscosity detectors. Biomacromolecules 2006, 7, 2136–2146. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Tomren, H.B.; Christensen, B.E. Periodate oxidized alginates: Depolymerization kinetics. Carbohydr. Polym. 2011, 86, 1595–1601. [Google Scholar] [CrossRef]
- Gomez, C.G.; Rinaudo, M.; Villar, M.A. Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 2007, 67, 296–304. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Schirmer, B.C.; Aachmann, F.L.; Skjåk-Bræk, G.; Draget, K.I.; Christensen, B.E. Novel alginates prepared by independent control of chain stiffness and distribution of G-residues: Structure and gelling properties. Carbohydr. Polym. 2009, 77, 725–735. [Google Scholar] [CrossRef]
- Omtvedt, L.A.; Dalheim, M.Ø.; Nielsen, T.T.; Larsen, K.L.; Strand, B.L.; Aachmann, F.L. Efficient Grafting of Cyclodextrin to Alginate and Performance of the Hydrogel for Release of Model Drug. Sci. Reports 2019. submitted. [Google Scholar]
- Kristiansen, K.A.; Ballance, S.; Potthast, A.; Christensen, B.E. An evaluation of tritium and fluorescence labelling combined with multi-detector SEC for the detection of carbonyl groups in polysaccharides. Carbohydr. Polym. 2009, 76, 196–205. [Google Scholar] [CrossRef]
- Stanisci, A.; Aarstad, O.A.; Tøndervik, A.; Sletta, H.; Dypås, L.B.; Skjåk-Bræk, G.; Aachmann, F.L. Overall size of mannuronan C5-Epimerases influences their ability to epimerize modified alginates and alginate gels. Carbohydr. Polym. 2018, 180, 256–263. [Google Scholar] [CrossRef]
- Grasdalen, H. High-field, 1H-n.m.r. spectroscopy of alginate: Sequential structure and linkage conformations. Carbohydr. Res. 1983, 118, 255–260. [Google Scholar] [CrossRef]
- Grasdalen, H.; Larsen, B.; Smidsrød, O. A p.m.r. study of the composition and sequence of uronate residues in alginates. Carbohydr. Res. 1979, 68, 23–31. [Google Scholar] [CrossRef]
- Dalheim, M.Ø.; Ulset, A.-S.T.; Jenssen, I.B.; Christensen, B.E. Degradation kinetics of peptide-coupled alginates prepared via the periodate oxidation reductive amination route. Carbohydr. Polym. 2017, 157, 1844–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draget, K.I.; Østgaard, K.; Smidsrød, O. Homogeneous alginate gels: A technical approach. Carbohydr. Polym. 1990, 14, 159–178. [Google Scholar] [CrossRef]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Painter, T.; Larsen, B. Formation of Hemiacetals between Neighbouring Hexuronic Acid Residues during the Periodate Oxidation of Alginate. Acta Chem. Scand. 1970, 24, 813–833. [Google Scholar] [CrossRef]
- Stokke, B.T.; Smidsrød, O.; Zanetti, F.; Strand, W.; Skjåk-Bræk, G. Distribution of uronate residues in alginate chains in relation to alginate gelling properties—2: Enrichment of β-d-mannuronic acid and depletion of α-l-guluronic acid in sol fraction. Carbohydr. Polym. 1993, 21, 39–46. [Google Scholar] [CrossRef]
- Bowman, K.A.; Aarstad, O.A.; Nakamura, M.; Stokke, B.T.; Skjåk-Bræk, G.; Round, A.N. Single molecule investigation of the onset and minimum size of the calcium-mediated junction zone in alginate. Carbohydr. Polym. 2016, 148, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Stokke, B.T.; Draget, K.I.; Smidsrød, O.; Yuguchi, Y.; Urakawa, H.; Kajiwara, K. Small-Angle X-ray Scattering and Rheological Characterization of Alginate Gels. 1. Ca−Alginate Gels. Macromolecules 2000, 33, 1853–1863. [Google Scholar] [CrossRef]
- Yuguchi, Y.; Hasegawa, A.; Padoł, A.M.; Draget, K.I.; Stokke, B.T. Local structure of Ca2+ induced hydrogels of alginate–oligoguluronate blends determined by small-angle-X-ray scattering. Carbohydr. Polym. 2016, 152, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Skjåk-Bræk, G.; Donati, I.; Paoletti, S. Alginate Hydrogels: Properties and Applications. In Polysaccharide Hydrogels: Characterization and Biomedical Applications; Pan Stanford: Singapore, 2015; pp. 449–498. [Google Scholar]
- Martinsen, A.; Skjåk-Bræk, G.; Smidsrød, O. Alginate as immobilization material: I. Correlation between chemical and physical properties of alginate gel beads. Biotechnol. Bioeng. 1989, 33, 79–89. [Google Scholar] [CrossRef]
- Draget, K.I.; Gåserød, O.; Aune, I.; Andersen, P.O.; Storbakken, B.; Stokke, B.T.; Smidsrød, O. Effects of molecular weight and elastic segment flexibility on syneresis in Ca–alginate gels. Food Hydrocoll. 2001, 15, 485–490. [Google Scholar] [CrossRef]
- Aarstad, O.; Heggset, B.E.; Pedersen, S.I.; Bjørnøy, H.S.; Syverud, K.; Strand, L.B. Mechanical Properties of Composite Hydrogels of Alginate and Cellulose Nanofibrils. Polymers 2017, 9, 378. [Google Scholar] [CrossRef]
- Smidsrød, O.; Moe, S.T. Biopolymer Chemistry; Postmyr, L., Ed.; Tapir Academic Press: Trondheim, Norway, 2008. [Google Scholar]
- Nielsen, T.T.; Wintgens, V.; Amiel, C.; Wimmer, R.; Larsen, K.L. Facile Synthesis of beta-Cyclodextrin-Dextran Polymers by “Click” Chemistry. Biomacromolecules 2010, 11, 1710–1715. [Google Scholar] [CrossRef]
- Smidsrød, O.; Haug, A.; Lian, B. Properties of Poly(1,4-hexuronates) in the Gel State. I. Evaluation of a Method for the Determination of Stiffness. Acta Chem. Scand. 1972, 26, 71–78. [Google Scholar] [CrossRef]
- Ertesvåg, H.; Skjåk-Bræk, G. Modification of Alginate Using Mannuronan C-5-Epimerases.; Bucke, C., Ed.; Humana Press: Totowa, NJ, USA, 1999. [Google Scholar]







| Material | P0 | DS (%) | Mw (kDa) |
|---|---|---|---|
| L. hyperborea stipe alginate | 0.00 | - | 133 |
| POA | 0.02 | - | 99 |
| POA | 0.04 | - | 93 |
| POA | 0.08 | - | 97 |
| POA-MeOTyr | 0.08 | 7.0 | 114 |
| POA-β-CyD | 0.08 | 1.6 | 65 |
| POA-GRGDSP | 0.08 | 3.9 | 134 |
| Epim POA-MeOTyr | 0.08 | 7.9 | 126 |
| Alginate | POA | POA-MeOTyr | POA-β-CyD | Epim POA-MeOTyr |
|---|---|---|---|---|
| Young’s modulus, E (kPa) | 1.2 ± 0.5 | 1.9 ± 0.7 | 0.7 ± 0.1 | 2.5 ± 0.3 |
| Syneresis (%) | 67 ± 7 | 46 ± 6 | 59 ± 3 | 54 ± 1 |
| Stress at rupture (kg) | 0.29 ± 0.06 | 0.55 ± 0.04 | 0.13 ± 0.01 | 3.01 ± 0.04 |
| Deformation at rupture (%) | 52 ± 3 | 60 ± 2 | 46 ± 3 | 63 ± 2 |
| Alginate | Mw (kDa) | FG | FM | FGG | FMG/FGM | FMM | FMGG/FGGM | FMGM | FGGG | NG>1 |
|---|---|---|---|---|---|---|---|---|---|---|
| L. hyperborea stipe alginate | 133 | 0.65 | 0.35 | 0.53 | 0.12 | 0.23 | 0.05 | 0.10 | 0.48 | 11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalheim, M.Ø.; Omtvedt, L.A.; Bjørge, I.M.; Akbarzadeh, A.; Mano, J.F.; Aachmann, F.L.; Strand, B.L. Mechanical Properties of Ca-Saturated Hydrogels with Functionalized Alginate. Gels 2019, 5, 23. https://doi.org/10.3390/gels5020023
Dalheim MØ, Omtvedt LA, Bjørge IM, Akbarzadeh A, Mano JF, Aachmann FL, Strand BL. Mechanical Properties of Ca-Saturated Hydrogels with Functionalized Alginate. Gels. 2019; 5(2):23. https://doi.org/10.3390/gels5020023
Chicago/Turabian StyleDalheim, Marianne Ø., Line Aa. Omtvedt, Isabel M. Bjørge, Anita Akbarzadeh, João F. Mano, Finn L. Aachmann, and Berit L. Strand. 2019. "Mechanical Properties of Ca-Saturated Hydrogels with Functionalized Alginate" Gels 5, no. 2: 23. https://doi.org/10.3390/gels5020023