Validation of an Analytical Method for Nitrite and Nitrate Determination in Meat Foods for Infants by Ion Chromatography with Conductivity Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
2.2. Instrument Setup
2.3. Standard, Sample, and Solvent Preparation
2.4. Validation Procedure
3. Results and Discussion
3.1. Chromatographic Separation
3.2. Method Validation
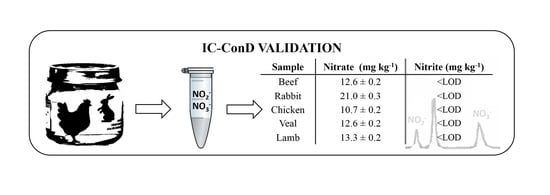
3.3. Sample Analysis
3.4. Daily Intake
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopez-Moreno, C.; Perez, I.V.; Urbano, A.M. Development and validation of an ionic chromatography method for the determination of nitrate, nitrite and chloride in meat. Food Chem. 2016, 194, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.M.; Steinberg, V.E. Health implications of nitrate and nitrite in drinking water: An update on methemoglobinemia occurrence and reproductive and developmental toxicity. Regul. Toxicol. Pharmacol. 1996, 23, 35–43. [Google Scholar] [CrossRef]
- Cammack, R.; Joannou, C.L.; Cui, X.Y.; Torres Martinez, C.; Maraj, S.R.; Hughes, M.N. Nitrite and nitrosyl compounds in food preservation. Biochim. Biophys. Acta Bioenerg. 1999, 1411, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Butler, A. Nitrites and nitrates in the human diet: Carcinogens or beneficial hypotensive agents? J. Ethnopharmacol. 2015, 167, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Caivano, M.; Pascale, R.; Mazzone, G.; Buchicchio, A.; Masi, S.; Bianco, G.; Caniani, D. N2O and CO2 Emissions from secondary settlers in WWTPs: Experimental results on full and pilot scale plants. In Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2017; pp. 412–418. [Google Scholar]
- Caivano, M.; Pascale, R.; Mazzone, G.; Masi, S.; Panariello, S.; Caniani, D. Disinfection unit of water resource recovery facilities: Critical issue for N2O Emission. In Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2017; pp. 444–450. [Google Scholar]
- Wang, Q.H.; Yu, L.J.; Liu, Y.; Lin, L.; Lu, R.G.; Zhu, J.P.; He, L.; Lu, Z.L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Jeddi, S.; Azizi, F.; Ghasemi, A.; Hadaegh, F. Nitrate and nitrite content of vegetables, fruits, grains, legumes, dairy products, meats and processed meats. J. Food Compos. Anal. 2016, 51, 93–105. [Google Scholar] [CrossRef]
- Cortesi, M.L.; Vollano, L.; Peruzy, M.F.; Marrone, R.; Mercogliano, R. Determination of nitrate and nitrite levels in infant foods marketed in Southern Italy. CYTA J. Food 2015, 13, 629–634. [Google Scholar] [CrossRef]
- Cox, R.D. Determination Of Nitrate And Nitrite At The Parts Per Billion Level By Chemiluminescence. Anal. Chem. 1980, 52, 332–335. [Google Scholar] [CrossRef]
- Zhao, K.; Song, H.; Zhuang, S.; Dai, L.; He, P.; Fang, Y. Determination of nitrite with the electrocatalytic property to the oxidation of nitrite on thionine modified aligned carbon nanotubes. Electrochem. Commun. 2007, 9, 65–70. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Silva, S. Quantification of residual nitrite and nitrate in ham by reverse-phase high performance liquid chromatography/diode array detector. Talanta 2008, 74, 1598–1602. [Google Scholar] [CrossRef]
- Oztekin, N.; Nutku, M.S.; Erim, F.B. Simultaneous determination of nitrite and nitrate in meat products and vegetables by capillary electrophoresis. Food Chem. 2002, 76, 103–106. [Google Scholar] [CrossRef]
- Biswas, S.; Chowdhury, B.; Ray, B.C. A novel spectrofluorimetric method for the ultra trace analysis of nitrite and nitrate in aqueous medium and its application to air, water, soil and forensic samples. Talanta 2004, 64, 308–312. [Google Scholar] [CrossRef]
- Yin, X.; Chen, Q.; Song, H.; Yang, M.; Wang, H. Sensitive and selective electrochemiluminescent detection of nitrite using dual-stabilizer-capped CdTe quantum dots. Electrochem. Commun. 2013, 34, 81–85. [Google Scholar] [CrossRef]
- Helaleh, M.I.; Korenaga, T. Ion chromatographic method for simultaneous determination of nitrate and nitrite in human saliva. J. Chromatogr. B Biomed. Sci. Appl. 2000, 744, 433–437. [Google Scholar] [CrossRef]
- Ventura, G.; Calvano, C.D.; Losito, I.; Bianco, G.; Pascale, R.; Palmisano, F.; Cataldi, T.R.I. Effect of pH and mobile phase additives on the chromatographic behaviour of an amide-embedded stationary phase: Cyanocobalamin and its diaminemonochloro-platinum (II) conjugate as a case study. J. Sep. Sci. 2019, 42, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, T.R.I.; Orlando, D.; Nardiello, D.; Rubino, A.; Bianco, G.; Abate, S.; Ciriello, R.; Guerrieri, A. A three-factor Doehlert matrix design in optimising the determination of octadecyltrimethylammonium bromide by cation-exchange chromatography with suppressed conductivity detection. Anal. Chim. Acta 2007, 597, 129–136. [Google Scholar] [CrossRef]
- Genualdi, S.; Jeong, N.; DeJager, L. Determination of endogenous concentrations of nitrites and nitrates in different types of cheese in the United States: Method development and validation using ion chromatography. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Iammarino, M.; Di Taranto, A.; Cristino, M. Monitoring of nitrites and nitrates levels in leafy vegetables (spinach and lettuce): A contribution to risk assessment. J. Sci. Food Agric. 2014, 94, 773–778. [Google Scholar] [CrossRef]
- Saccani, G.; Gherardi, S.; Trifirò, A.; Soresi Bordini, C.; Calza, M.; Freddi, C. Use of ion chromatography for the measurement of organic acids in fruit juices. J. Chromatogr. A 1995, 706, 395–403. [Google Scholar] [CrossRef]
- Siu, D.C.; Henshall, A. Ion chromatographic determination of nitrate and nitrite in meat products. J. Chromatogr. A 1998, 804, 157–160. [Google Scholar] [CrossRef]
- Merino, L.; Edberg, U.; Fuchs, G.; Åman, P. Liquid chromatographic determination of residual nitrite/nitrate in foods: NMKL collaborative study. J. AOAC Int. 2000, 83, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMullen, S.E.; Casanova, J.A.; Gross, L.K.; Schenck, F.J. Ion chromatographic determination of nitrate and nitrite in vegetable and fruit baby foods. J. AOAC Int. 2005, 8, 1793–1796. [Google Scholar] [CrossRef] [Green Version]
- European Commission. COMMISSION REGULATION (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union Bruss., L 2006, 364/5. [Google Scholar] [CrossRef]
- Gates, K.; Chang, N.; Dilek, I.; Jian, H.; Pogue, S.; Sreenivasan, U. The uncertainty of reference standards—A guide to understanding factors impacting uncertainty, uncertainty calculations, and vendor certifications. J. Anal. Toxicol. 2009, 33, 532–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eurachem. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics. Magnusson, B., Örnemark, U., Eds.; 2014. Available online: https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf (accessed on 26 July 2020).
- Eurachem. EURACHEM/CITAC Guide: Quantifying Uncertainty in Analytical Measurement; Ellison, S.L.R., Williams, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ter Heine, R.; Rosing, H.; Van Gorp, E.C.M.; Mulder, J.W.; Van der Steeg, W.A.; Beijnen, J.H.; Huitema, A.D.R. Quantification of protease inhibitors and non-nucleoside reverse transcriptase inhibitors in dried blood spots by liquid chromatography-triple quadrupole mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 867, 205–212. [Google Scholar] [CrossRef]
- ICH. ICH harmonised tripartite guideline validation of analytical procedures: Text and methodology Q2(R1). Guidance 2005, 1, 1–15. [Google Scholar]
- AOAC. International Guidelines for single laboratory validation of chemical methods for dietary supplements and botanicals. AOAC Int. 2002, 12–19. [Google Scholar]
- Chamandust, S.; Mehrasebi, M.R.; Kamali, K.; Solgi, R.; Taran, J.; Nazari, F.; Hosseini, M.-J. Simultaneous Determination of Nitrite and Nitrate in Milk Samples by Ion Chromatography Method and Estimation of Dietary Intake. Int. J. Food Prop. 2016, 19, 1983–1993. [Google Scholar] [CrossRef]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC Trends Anal. Chem. 2004, 23, 480–490. [Google Scholar] [CrossRef]
- Moorcroft, M. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- European Commission. Opinion on Nitrate and Nitrite. Reports of the Scientific Committee for Food (Thirty Eighth Series); Commission of the European Communities: Luxembourg, 1997. [Google Scholar]
- Speijers, G.; Van den Brandt, P.A. Nitrite and potential endogenous formation of N-nitroso compounds; safety evaluation of certain food additives, JECFA. WHO Food Addit. Ser. 2003, 50, 49–74. [Google Scholar]
- Özdestan, Ö.; Üren, A. Nitrate and Nitrite Contents of Baby Foods. Acad. Food J. 2012, 10, 11–18. [Google Scholar]
- Charrondiere, R.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Grilli, S.; Gürtler, R.; König, J.; Lambré, C.; Larsen, J.-C.; et al. Statement on nitrites in meat products. Panel Memb. F Aguilar U EFSA J. 2010, 8, 1538. [Google Scholar]
- Kersting, M.; Alexy, U.; Sichert-Hellert, W.; Manz, F.; Schöch, G. Measured consumption of commercial infant food products in German infants: Results from the DONALD study. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Vasco, E.R.; Alvito, P.C. Occurrence and infant exposure assessment of nitrates in baby foods marketed in the region of Lisbon, Portugal. Food Addit. Contam. Part B Surveill. 2011, 4, 218–225. [Google Scholar] [CrossRef]



| Quality Parameters | Analyte | ||
|---|---|---|---|
| Nitrite | Nitrate | ||
| Linearity | Linear range (mg L−1) | 0.456–3.57 | 0.472–3.76 |
| Regression equation, y = (m ± sm)x + (b ± sb) | y = (17.3 ± 0.3)10−3x + (0.9 ± 0.7)10−3 | y = (14.6 ± 0.1)10−3x + (1.4 ± 0.3)10−3 | |
| Determination coefficient (R2) | 0.9993 | 0.9998 | |
| Tcal (T0.05,4 = 2.78, k = 6) | 53.43 | 99.99 | |
| Precision, %RSD (concentration level, mg L−1) | Repeatability | 0.2 (0.456) | 0.04 (0.472) |
| 0.2 (0.893) | 0.04 (0.945) | ||
| 0.6 (1.79) | 0.1 (1.89) | ||
| 1.2 (3.57) | 0.4 (3.78) | ||
| Intermediate precision | 0.1 (0.456) | 0.2 (0.472) | |
| 0.2 (0.893) | 0.1 (0.945) | ||
| 0.4 (1.79) | 0.1 (1.89) | ||
| 1.0 (3.57) | 0.2 (3.78) | ||
| Trueness | %R (concentration, mg L−1) | 95 ± 2% (0.6) | 84 ± 6% (0.6) |
| 112 ± 11% (1.2) | 87 ± 9% (1.2) | ||
| 114 ± 6% (2.4) | 103 ± 2% (2.4) | ||
| Matrix effect (slope difference %) | 2.96 | 13.79 | |
| LOD (mg L−1) | 0.13 | 0.08 | |
| LOQ (mg L−1) | 0.44 | 0.26 | |
| Uncertainty, U (mg kg−1) (concentration level, mg L−1) | 0.2 (0.6) | ||
| 0.3 (1.2) | |||
| 0.5 (2.4) | |||
| Sample | Nitrate (mg kg−1) | Nitrite (mg kg−1) |
|---|---|---|
| Beef | 12.6 ± 0.2 | <LOD |
| Rabbit | 21.0 ± 0.3 | <LOD |
| Chicken | 10.7 ± 0.2 | <LOD |
| Veal | 12.6 ± 0.2 | <LOD |
| Lamb | 13.3 ± 0.2 | <LOD |
| Consumption Scenarios | Estimation of Nitrate | % ADI | ||||||
|---|---|---|---|---|---|---|---|---|
| Age (Months) | Body Weight (kg) | Mean Consumption of Baby Foods (g day−1) | Mean (14.04 mg kg−1) | Highest Value (21.0 mg kg−1) | Lowest Value (10.7 mg kg−1) | Mean (14.04 mg kg−1) | Highest Value (21.0 mg kg−1) | Lowest Value (10.7 mg kg−1) |
| 3 | 5.80 | 67.0 | 0.16 | 0.24 | 0.12 | 4.38 | 6.56 | 3.34 |
| 6 | 7.50 | 195.0 | 0.36 | 0.55 | 0.28 | 9.87 | 14.76 | 7.52 |
| 9 | 8.60 | 234.0 | 0.38 | 0.57 | 0.29 | 10.32 | 15.44 | 7.87 |
| 12 | 9.40 | 208.0 | 0.31 | 0.46 | 0.24 | 8.40 | 12.56 | 6.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coviello, D.; Pascale, R.; Ciriello, R.; Salvi, A.M.; Guerrieri, A.; Contursi, M.; Scrano, L.; Bufo, S.A.; Cataldi, T.R.I.; Bianco, G. Validation of an Analytical Method for Nitrite and Nitrate Determination in Meat Foods for Infants by Ion Chromatography with Conductivity Detection. Foods 2020, 9, 1238. https://doi.org/10.3390/foods9091238
Coviello D, Pascale R, Ciriello R, Salvi AM, Guerrieri A, Contursi M, Scrano L, Bufo SA, Cataldi TRI, Bianco G. Validation of an Analytical Method for Nitrite and Nitrate Determination in Meat Foods for Infants by Ion Chromatography with Conductivity Detection. Foods. 2020; 9(9):1238. https://doi.org/10.3390/foods9091238
Chicago/Turabian StyleCoviello, Donatella, Raffaella Pascale, Rosanna Ciriello, Anna Maria Salvi, Antonio Guerrieri, Michela Contursi, Laura Scrano, Sabino A. Bufo, Tommaso R.I. Cataldi, and Giuliana Bianco. 2020. "Validation of an Analytical Method for Nitrite and Nitrate Determination in Meat Foods for Infants by Ion Chromatography with Conductivity Detection" Foods 9, no. 9: 1238. https://doi.org/10.3390/foods9091238