Effect of Different Cooking Methods on Proton Dynamics and Physicochemical Attributes in Spanish Mackerel Assessed by Low-Field NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cooking of Fish Samples
2.2. H Low-Field Nuclear Magnetic Resonance (LF-NMR) and Magnetic Resonance Imaging (MRI) Analysis
2.3. Cooking Loss and WHC Measurements
2.4. Color Analysis
2.5. Textural Profile Analysis (TPA)
2.6. Extraction of Myofibrillar Protein (MP) from Spanish Mackerel Meat
2.7. Surface Hydrophobicity of the MP
2.8. Total Carbonyl Content
2.9. Free Thiols Measurement
2.10. Lipid Oxidation
2.11. Cryo-Scanning Electron Microscopy (Cryo-SEM)
2.12. Circular Dichroism (CD)
2.13. Statistical Analysis
3. Results
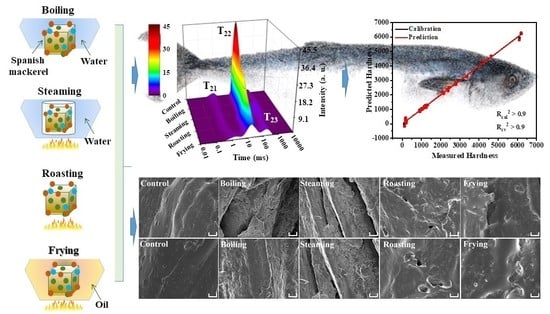
3.1. Proton Dynamics during Boiling, Steaming, Roasting, Frying Assessed by LF-NMR
3.2. Cooking Loss, Water-Holding Capacity Analysis
3.3. Color Analysis
3.4. Protein and Lipid Oxidation Characterization
3.5. CD Analysis
3.6. Cryo-SEM Spectra Analysis
3.7. Principal Component Analysis (PCA) of NMR Parameters
3.8. Texture Profile Analysis
3.9. Partial Least Squares (PLS) Regression Models
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.-R.; Wang, B.; Chi, C.-F.; Zhang, Q.-H.; Gong, Y.-D.; Tang, J.-J.; Luo, H.-Y.; Ding, G.-F. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocolloids 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Garcıa-Arias, M.T.; Pontes, E.Á.; Garcıa-Linares, M.C.; García-Fernández, M.C.; Sánchez-Muniz, F.J. Cooking–freezing–reheating (CFR) of sardine (Sardina pilchardus) fillets. Effect of different cooking and reheating procedures on the proximate and fatty acid compositions. Food Chem. 2003, 83, 349–356. [Google Scholar] [CrossRef]
- Geng, S.; Wang, H.; Wang, X.; Ma, X.; Xiao, S.; Wang, J.; Tan, M. A non-invasive NMR and MRI method to analyze the rehydration of dried sea cucumber. Anal. Methods 2015, 7, 2413–2419. [Google Scholar] [CrossRef]
- Kirtil, E.; Cikrikci, S.; McCarthy, M.J.; Oztop, M.H. Recent advances in time domain NMR & MRI sensors and their food applications. Curr. Opin. Food Sci. 2017, 17, 9–15. [Google Scholar]
- Li, M.; Li, B.; Zhang, W. Rapid and non-invasive detection and imaging of the hydrocolloid-injected prawns with low-field NMR and MRI. Food Chem. 2018, 242, 16–21. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Lin, Z.; Tan, M. Differences between constant and intermittent drying in surf clam: Dynamics of water mobility and distribution study. Dry. Technol. 2017, 36, 1273–1283. [Google Scholar] [CrossRef]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Effect of heat treatment on changes in texture, structure and properties of Thai indigenous chicken muscle. Food Chem. 2005, 93, 337–348. [Google Scholar] [CrossRef]
- Marimuthu, K.; Thilaga, M.; Kathiresan, S.; Xavier, R.; Mas, R.H. Effect of different cooking methods on proximate and mineral composition of striped snakehead fish (Channa striatus, Bloch). J. Food Sci. Technol. 2012, 49, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M.; Jarvis, M.C.; Duncan, H.J. The textural analysis of cooked potato. 3. Simple methods for determining texture. Potato Res. 1992, 35, 103–109. [Google Scholar] [CrossRef]
- Hansen, C.L.; Thybo, A.K.; Bertram, H.C.; Viereck, N.; van den Berg, F.; Engelsen, S.B. Determination of dry matter content in potato tubers by low-field nuclear magnetic resonance (LF-NMR). J. Agric. Food Chem. 2010, 58, 10300–10304. [Google Scholar] [CrossRef]
- Tan, M.; Lin, Z.; Zu, Y.; Zhu, B.; Cheng, S. Effect of multiple freeze-thaw cycles on the quality of instant sea cucumber: Emphatically on water status of by LF-NMR and MRI. Food Res. Int. 2018, 109, 65–71. [Google Scholar] [CrossRef]
- Chen, H.H.; Chiu, E.M.; Huang, J.R. Color and gel-forming properties of horse mackerel (Trachurus japonicus) as related to washing conditions. J. Food Sci. 1997, 62, 985–991. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Xiong, Y.; Ren, Y. Decreased gelling and emulsifying properties of myofibrillar protein from repeatedly frozen-thawed porcine longissimus muscle are due to protein denaturation and susceptibility to aggregation. Meat Sci. 2010, 85, 481–486. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, M.; Tang, C.; Han, M.; Xu, X.; Zhou, G. Glycation-induced structural modification of myofibrillar protein and its relation to emulsifying properties. LWT-Food Sci. Technol. 2020, 117, 108664. [Google Scholar] [CrossRef]
- Wang, S.; Lin, R.; Cheng, S.; Wang, Z.; Tan, M. Assessment of water mobility in surf clam and soy protein system during gelation using LF-NMR technique. Foods 2020, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Mercier, Y.; Gatellier, P.; Viau, M.; Remignon, H.; Renerre, M. Effect of dietary fat and vitamin E on colour stability and on lipid and protein oxidation in turkey meat during storage. Meat Sci. 1998, 48, 301–318. [Google Scholar] [CrossRef]
- Morzel, M.; Gatellier, P.; Sayd, T.; Renerre, M.; Laville, E. Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Sci. 2006, 73, 536–543. [Google Scholar] [CrossRef]
- John, L.; Cornforth, D.; Carpenter, C.E.; Sorheim, O.; Pettee, B.C.; Whittier, D.R. Color and thiobarbituric acid values of cooked top sirloin steaks packaged in modified atmospheres of 80% oxygen, or 0.4% carbon monoxide, or vacuum. Meat Sci. 2005, 69, 441–449. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Li, R.; Yang, H.; Wang, H.; Wang, H.; Tan, M. Influence of multiple freeze-thaw cycles on quality characteristics of beef semimembranous muscle: With emphasis on water status and distribution by LF-NMR and MRI. Meat Sci. 2019, 147, 44–52. [Google Scholar] [CrossRef]
- Ogawa, M.; Nakamura, S.; Horimoto, Y.; An, H.; Tsuchiya, T.; Nakai, S. Raman spectroscopic study of changes in fish actomyosin during setting. J. Agric. Food Chem. 1999, 47, 3309–3318. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Moreno, P.; Careche, M. Low field nuclear magnetic resonance (LF-NMR) relaxometry in hake (Merluccius merluccius, L.) muscle after different freezing and storage conditions. Food Chem. 2014, 153, 250–257. [Google Scholar] [CrossRef]
- Aursand, I.G.; Veliyulin, E.; Böcker, U.; Ofstad, R.; Rustad, T.; Erikson, U. Water and salt distribution in Atlantic salmon (Salmo salar) studied by low-field 1H NMR, 1H and 23Na MRI and light microscopy: Effects of raw material quality and brine salting. J. Agric. Food Chem. 2008, 57, 46–54. [Google Scholar] [CrossRef]
- Xia, K.; Xu, W.; Huang, L.; Song, Y.; Zhu, B.W.; Tan, M. Water dynamics of turbot flesh during frying, boiling, and stewing processes and its relationship with color and texture properties: Low-field NMR and MRI studies. J. Food Process. Preserv. 2018, 42, e13338. [Google Scholar] [CrossRef] [Green Version]
- Aroeira, C.N.; de Almeida Torres Filho, R.; Fontes, P.R.; de Lemos Souza Ramos, A.; de Miranda Gomide, L.A.; Ladeira, M.M.; Ramos, E.M. Effect of freezing prior to aging on myoglobin redox forms and CIE color of beef from Nellore and Aberdeen Angus cattle. Meat Sci. 2017, 125, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Ren, S.; Shen, Q.; Chen, J.; Ye, X.; Ling, J. Proteomic study of the effect of different cooking methods on protein oxidation in fish fillets. RSC Adv. 2017, 7, 27496–27505. [Google Scholar] [CrossRef] [Green Version]
- Traore, S.; Aubry, L.; Gatellier, P.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Santé-Lhoutellier, V. Effect of heat treatment on protein oxidation in pig meat. Meat Sci. 2012, 91, 14–21. [Google Scholar] [CrossRef]
- Li, T.; Wang, C.; Li, T.; Ma, L.; Sun, D.; Hou, J.; Jiang, Z. Surface hydrophobicity and functional properties of citric acid cross-linked whey protein isolate: The impact of pH and concentration of citric acid. Molecules 2018, 23, 2383. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Andrés, J.M.; de Alba, M.; Harrison, S.M.; Brunton, N.P.; Cullen, P.; Tiwari, B.K. Effects of cold atmospheric plasma on mackerel lipid and protein oxidation during storage. LWT-Food Sci. Technol. 2020, 118, 108697. [Google Scholar] [CrossRef]
- Guyon, C.; Meynier, A.; de Lamballerie, M. Protein and lipid oxidation in meat: A review with emphasis on high-pressure treatments. Trends Food Sci. Tech. 2016, 50, 131–143. [Google Scholar] [CrossRef]
- Şener, E.; Yildiz, M.; Savaş, E. Effects of dietary lipids on growth and fatty acid composition in Russian sturgeon (Acipenser gueldenstaedtii) juveniles. Turk. J. Vet. Anim. Sci. 2005, 29, 1101–1107. [Google Scholar]
- Gavin, C.; Verbeek, C.J.R.; Lay, M.C.; Bier, J.M.; Hicks, T.M. Thermal analysis and secondary structure of protein fractions in a highly aggregated protein material. Polym. Test. 2019, 77, 105876. [Google Scholar] [CrossRef]
- Bier, J.M.; Verbeek, C.J.R.; Lay, M.C. Using synchrotron FTIR spectroscopy to determine secondary structure changes and distribution in thermoplastic protein. J. Appl. Polym. Sci. 2013, 130, 359–369. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Piñeiro, C.; Gallardo, J.M.; Barros-Velazquez, J. Biochemical changes and quality loss during chilled storage of farmed turbot (Psetta maxima). Food Chem. 2005, 90, 445–452. [Google Scholar] [CrossRef]
- Barbosa, L.L.; Sad, C.M.S.; Morgan, V.G.; Santos, M.F.P.; Castro, E.V.R. Time-domain proton nuclear magnetic resonance and chemometrics for identification and classification of Brazilian petroleum. Energy Fuels 2013, 27, 6560–6566. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Li, X.; Zhong, K.; Li, Y.; Li, J. Effects of different freezing treatments on physicochemical responses and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) fillets during refrigerated storage. LWT-Food Sci. Technol. 2014, 59, 122–129. [Google Scholar] [CrossRef]
- Qi, J.; Li, C.; Chen, Y.; Gao, F.; Xu, X.; Zhou, G. Changes in meat quality of ovine longissimus dorsi muscle in response to repeated freeze and thaw. Meat Sci. 2012, 92, 619–626. [Google Scholar] [CrossRef]
- Chen, T.H.; Zhu, Y.P.; Han, M.Y.; Wang, P.; Wer, R.; Xu, X.; Zhou, G. Classification of chicken muscle with different freeze–thaw cycles using impedance and physicochemical properties. J. Food Eng. 2017, 196, 94–100. [Google Scholar] [CrossRef]








| Treatment | T21 (ms) | T22 (ms) | T23 (ms) | A21(g−1) | A22 (g−1) | A23 (g−1) |
|---|---|---|---|---|---|---|
| Control | 1.01 ± 0.05b | 51.04 ± 1.99a | 429.96 ± 42.12b | 526.50 ± 102.29a | 9577.17 ± 682.30a | 180.82 ± 57.56d |
| Boiling | 0.85 ± 0.08b | 30.21 ± 1.56b | 533.10 ± 56.36a | 341.55 ± 15.43c | 5667.80 ± 281.15b | 546.17 ± 227.15c |
| Steaming | 0.77 ± 0.09b | 26.60 ± 0.46c | 275.61 ± 14.37c | 307.60 ± 15.74c | 5261.56 ± 198.75b | 843.55 ± 117.85b |
| Roasting | 0.66 ± 0.13b | 18.01 ± 1.57d | 228.71 ± 20.67d | 355.09 ± 42.90c | 4162.73 ± 891.11c | 299.38 ± 126.77d |
| Frying | 1.44 ± 0.43a | 17.43 ± 2.50d | 142.94 ± 16.45e | 470.28 ± 100.14b | 1707.53 ± 399.69d | 1113.33 ± 235.26a |
| Oxidation Indexes | Treatments | ||||
|---|---|---|---|---|---|
| Control | Boiling | Steaming | Roasting | Frying | |
| Total carbonyl (nmol/mg) | 0.11 ± 0.05b | 0.12 ± 0.01b | 0.12 ± 0.01b | 0.17 ± 0.02b | 0.27 ± 0.08a |
| Free thiols (102 nmol/mg) | 4.62 ± 0.42a | 1.18 ± 0.13b | 1.02 ± 0.29b | 0.87 ± 0.01b | 0.57 ± 0.17b |
| Surface hydrophobicity | 54.89 ± 4.86a | 19.34 ± 0.18b | 20.67 ± 3.52b | 15.76 ± 0.03b | 14.88 ± 0.69b |
| TBARS (mg/kg) | 0.17 ± 0.01c | 0.26 ± 0.00b | 0.36 ± 0.02b | 0.64 ± 0.01a | 0.34 ± 0.06b |
| Processing | Hardness (g) | Resilience | Springiness | Chewiness | Gumminess | Adhesiveness |
|---|---|---|---|---|---|---|
| Control | 177.01 ± 11.50d | 0.23 ± 0.02b | 0.76 ± 0.03b | 87.6 ± 9.76d | 115.78 ± 10.28d | 0.65 ± 0.02a |
| Boiling | 1040.98 ± 120.72c | 0.24 ± 0.03b | 0.79 ± 0.03b | 493.22 ± 84.62c | 624.36 ± 94.01c | 0.60 ± 0.03b |
| Steaming | 996.51 ± 128.69c | 0.22 ± 0.01b | 0.80 ± 0.03b | 459.91 ± 70.94c | 576.04 ± 81.92c | 0.58 ± 0.03b |
| Roasting | 2399.01 ± 222.93b | 0.30 ± 0.04a | 0.87 ± 0.02a | 1430.15 ± 235.32b | 1643.56 ± 263.85b | 0.68 ± 0.07a |
| Frying | 6561.44 ± 623.91a | 0.25 ± 0.04b | 0.85 ± 0.05a | 3255.96 ± 464.38a | 3826.61 ± 349.78a | 0.59 ± 0.07b |
| Calibration | Validation | |||||
|---|---|---|---|---|---|---|
| PLS Factors | Rcal2 | RMSEC | Rcv2 | RMSECV | RPD (%) | |
| Hardness (g) | 7 | 0.997 | 77.679 | 0.995 | 109.128 | 119.389 |
| Resilience | 7 | 0.994 | 0.003 | 0.991 | 0.004 | 110.067 |
| Springiness | 7 | 0.990 | 0.006 | 0.984 | 0.008 | 69.282 |
| Chewiness | 7 | 0.997 | 44.047 | 0.994 | 59.683 | 97.453 |
| Gumminess | 7 | 0.997 | 49.245 | 0.994 | 67.477 | 100.154 |
| Adhesiveness | 6 | 0.989 | 0.007 | 0.983 | 0.009 | 84.817 |
| Texture Parameters | Treatment | Predicted Value | Measured Value | Recovery (%) | CV (n = 4, %) |
|---|---|---|---|---|---|
| Hardness | Control | 229.61 ± 7.56 | 191.84 ± 14.07 | 83.47 ± 0.04 | 4.86 |
| Boiling | 1310.50 ± 64.63 | 1256.00 ± 57.68 | 95.87 ± 0.02 | 2.38 | |
| Steaming | 1106.50 ± 41.49 | 1100.75 ± 42.19 | 99.52 ± 0.03 | 3.23 | |
| Roasting | 2317.50 ± 21.46 | 2384.75 ± 26.59 | 102.91 ± 0.01 | 1.27 | |
| Frying | 3838.50 ± 784.29 | 3761.50 ± 824.16 | 97.76 ± 0.02 | 1.62 | |
| Resilience | Control | 0.23 ± 0.00 | 0.23 ± 0.00 | 100.32 ± 0.01 | 0.89 |
| Boiling | 0.26 ± 0.01 | 0.26 ± 0.01 | 100.58 ± 0.00 | 0.23 | |
| Steaming | 0.23 ± 0.00 | 0.23 ± 0.00 | 101.52 ± 0.01 | 0.56 | |
| Roasting | 0.31 ± 0.02 | 0.31 ± 0.02 | 100.83 ± 0.01 | 0.76 | |
| Frying | 0.22 ± 0.01 | 0.22 ± 0.01 | 100.38 ± 0.01 | 1.42 | |
| Springiness | Control | 0.76 ± 0.01 | 0.75 ± 0.01 | 99.22 ± 0.02 | 1.68 |
| Boiling | 0.80 ± 0.01 | 0.81 ± 0.00 | 100.66 ± 0.01 | 0.98 | |
| Steaming | 0.79 ± 0.01 | 0.80 ± 0.00 | 100.51 ± 0.01 | 1.02 | |
| Roasting | 0.89 ± 0.01 | 0.89 ± 0.01 | 100.00 ± 0.00 | 0.28 | |
| Frying | 0.86 ± 0.01 | 0.86 ± 0.02 | 99.27 ± 0.01 | 1.18 | |
| Chewiness | Control | 120.60 ± 3.48 | 92.08 ± 3.06 | 76.38 ± 0.03 | 3.33 |
| Boiling | 629.33 ± 28.67 | 600.49 ± 27.50 | 95.46 ± 0.03 | 3.60 | |
| Steaming | 518.68 ± 19.97 | 528.69 ± 17.46 | 101.96 ± 0.02 | 1.81 | |
| Roasting | 1285.00 ± 91.08 | 1304.00 ± 94.53 | 101.49 ± 0.02 | 2.14 | |
| Frying | 1560.00 ± 310.47 | 1524.50 ± 319.07 | 97.59 ± 0.02 | 1.88 | |
| Gumminess | Control | 155.59 ± 7.23 | 121.93 ± 8.26 | 78.34 ± 0.03 | 3.58 |
| Boiling | 750.74 ± 44.10 | 734.73 ± 30.12 | 97.97 ± 0.03 | 3.30 | |
| Steaming | 643.96 ± 19.80 | 651.91 ± 9.37 | 101.29 ± 0.03 | 2.70 | |
| Roasting | 1638.50 ± 93.23 | 1676.75 ± 88.44 | 102.36 ± 0.02 | 1.72 | |
| Frying | 1858.75 ± 319.09 | 1816.25 ± 322.34 | 97.64 ± 0.02 | 1.67 | |
| Adhesiveness | Control | 0.64 ± 0.01 | 0.64 ± 0.01 | 100.40 ± 0.01 | 1.32 |
| Boiling | 0.63 ± 0.01 | 0.63 ± 0.02 | 100.44 ± 0.00 | 0.20 | |
| Steaming | 0.59 ± 0.02 | 0.60 ± 0.02 | 100.68 ± 0.00 | 0.15 | |
| Roasting | 0.67 ± 0.02 | 0.68 ± 0.02 | 100.18 ± 0.01 | 0.71 | |
| Frying | 0.54 ± 0.01 | 0.62 ± 0.01 | 113.57 ± 0.02 | 2.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Wang, S.; Lin, R.; Cheng, S.; Yuan, B.; Wang, Z.; Tan, M. Effect of Different Cooking Methods on Proton Dynamics and Physicochemical Attributes in Spanish Mackerel Assessed by Low-Field NMR. Foods 2020, 9, 364. https://doi.org/10.3390/foods9030364
Sun S, Wang S, Lin R, Cheng S, Yuan B, Wang Z, Tan M. Effect of Different Cooking Methods on Proton Dynamics and Physicochemical Attributes in Spanish Mackerel Assessed by Low-Field NMR. Foods. 2020; 9(3):364. https://doi.org/10.3390/foods9030364
Chicago/Turabian StyleSun, Shan, Siqi Wang, Rong Lin, Shasha Cheng, Biao Yuan, Zhixiang Wang, and Mingqian Tan. 2020. "Effect of Different Cooking Methods on Proton Dynamics and Physicochemical Attributes in Spanish Mackerel Assessed by Low-Field NMR" Foods 9, no. 3: 364. https://doi.org/10.3390/foods9030364