Bilberry and Sea Buckthorn Leaves and Their Subcritical Water Extracts Prevent Lipid Oxidation in Meat Products
Abstract
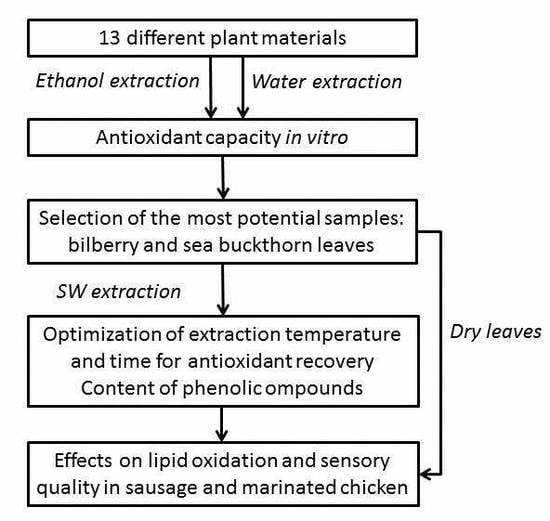
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Extraction of Antioxidants with Water and Ethanol-Water at Ambient Temperature
2.4. Two-Step Extraction of Tree Materials and Determination of Phenolic Compounds
2.5. Subcritical Water Extraction of Berry Leaves and Determination of Phenolic Compounds
2.6. Antioxidant Activity of Plant Extracts In Vitro
2.7. Application of Berry Leaves and Their Subcritical Water Extracts in Chicken Marinades and Pork Sausages
2.8. Sensory Evaluation
2.9. Oxidation of Lipids in the Meat Products
3. Results and Discussion
3.1. Antioxidant Activities of Water, Ethanolic (aq) and Hexane/Ethanol Extracts In Vitro
3.2. Subcritical Water Extraction for Bilberry and Sea Buckthorn Leaves and Antioxidant Activities of the Extracts
3.3. Polyphenol Contents in the Leaf and Tree Extracts
3.4. Antioxidative Effects of Berry Leaves and Their SW Extracts in Marinated Chicken and Pork Sausage
3.5. Effects of the Plant Ingredients on the Sensory Properties of the Meat Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohamed, H.M.H.; Mansour, H.A. Incorporating essential oils of marjoram and rosemary in the formulation of beef patties manufactured with mechanically deboned poultry meat to improve the lipid stability and sensory attributes. LWT Food Sci. Technol. 2012, 45, 79–87. [Google Scholar] [CrossRef]
- Devatkal, S.K.; Narsaiah, K.; Borah, A. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2010, 85, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Alvarez, R.; O’Sullivan, M.; Dowling, D.P.; Gargan, S.O.; Monahan, F.J. Effect of an active packaging with citrus extract on lipid oxidation and sensory quality of cooked turkey meat. Meat Sci. 2014, 96, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Bosco, S.J.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Fernandes, R.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, G.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Trindade, M.A.; Ahn, D.U.; Barba, F.J. Natural antioxidants to reduce the oxidation process of meat and meat products. Food Res. Int. 2019, 115, 377–378. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Tech. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 797–812. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Liimatainen, J.; Alanne, A.-N.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2016, 220, 266–281. [Google Scholar] [CrossRef]
- Ovaskainen, M.-L.; Törrönen, R.; Koponen, J.; Sinkko, H.; Hellström, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Jyske, T.; Laakso, T.; Latva-Mäenpää, H.; Tapanila, T.; Saranpää, P. Yield of stilbene glucosides from the bark of young and old Norway spruce stems. Biomass Bioenergy 2014, 71, 216–227. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Holmbom, B. Phenolic and Lipophilic Extractives in Scots Pine Knots and Stemwood. Holzforschung 2003, 57, 359–372. [Google Scholar] [CrossRef]
- Shitu, A.; Izhar, S.; Tahir, T.M. Sub-critical water as a green solvent for production of valuable materials from agricultural waste biomass: A review of recent work. GJESM 2015, 1, 255–264. [Google Scholar]
- Laavola, M.; Nieminen, R.; Leppanen, T.; Eckerman, C.; Holmbom, B.; Moilanen, E. Pinosylvin and Monomethylpinosylvin, Constituents of an Extract from the Knot of Pinus sylvestris, Reduce Inflammatory Gene Expression and Inflammatory Responses in Vivo. J. Agric. Food Chem. 2015, 63, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Örså, F.; Holmbom, B. A convenient method for determination of wood extractives in papermaking process waters and effluents. J. Pulp Pap. Sci. 1994, 20, J361–J366. [Google Scholar]
- Mattila, P.; Kumpulainen, J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef]
- Mattila, P.; Pihlava, J.-M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of phytochemicals and antinutritional factors in commercial protein-rich plant products. Food Qual. Saf. 2018, 2, 213–219. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, S.; Johansson, T.; Vegarud, G.E.; Pihlava, J.M.; Pihlanto, A. Angiotensin I-converting enzyme inhibitory and antioxidant properties of rapeseed hydrolysates. J. Funct. Foods 2012, 4, 575–583. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Valente, M.; Ferri, L.; Gregolin, C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim. Biophys. Acta 1982, 710, 197–211. [Google Scholar] [CrossRef]
- Tirmenstein, M.A.; Pierce, C.A.; Leraas, T.L.; Fariss, M.W.A. Fluoresence plate reader assay for monitoring the susceptibility of biological samples to lipid peroxidation. Anal. Biochem. 1998, 265, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Harper, H.H.; Hadley, M. Malondiladehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, E. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [Green Version]
- Tlili, N.; Elfalleh, W.; Hannachi, H.; Yahia, Y.; Khaldi, A.; Ferchichi, A.; Nasri, N. Screening of Natural Antioxidants from Selected Medicinal Plants. Int. J. Food Prop. 2013, 16, 1117–1126. [Google Scholar] [CrossRef]
- Viljanen, K.; Kylli, P.; Kivikari, R.; Heinonen, M. Inhibition of Protein and Lipid Oxidation in Liposomes by Berry Phenolics. J. Agric. Food Chem. 2004, 52, 7419–7424. [Google Scholar] [CrossRef]
- Apetrei, C.L.; Tuchilus, C.; Aprotosoaie, A.C.; Oprea, A.; Malterud, K.E.; Miron, A. Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. Bark and Needles. Molecules 2011, 16, 7773–7788. [Google Scholar] [CrossRef] [Green Version]
- Pietarinen, S.P.; Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood Sci. 2006, 52, 436–444. [Google Scholar] [CrossRef]
- Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Reunanen, M.H.; Eklund, P.C.; Sjöholm, R.E.; Eckerman, C.S.; Pohjamo, S.P.; Holmbom, B.R. Antioxidant activity of knotwood extractives and phenolic compounds of selected tree species. J. Agric. Food Chem. 2003, 51, 7600–7606. [Google Scholar] [CrossRef] [PubMed]
- Pihlanto, A.; Johansson, T.; Mäkinen, S. Inhibition of angiotensin I-converting enzyme and lipid peroxidation by fermented rapeseed and flaxseed meal. Eng. Life Sci. 2012, 12, 450–456. [Google Scholar] [CrossRef]
- Integrated Systems Laboratory Inc. Chemical Information Review Document for Arbutin [CAS No. 497-76-7] and Extracts from Arctostaphylos Uva-Ursi; Integrated Laboratory Systems, Inc.: Research Triangle Park, NC, USA, 2006; p. 38. [Google Scholar]
- Kumar, M.S.Y.; Dutta, R.; Prasad, D.; Misra, K. Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides) leaves for the comparative evaluation of antioxidant activity. Food Chem. 2011, 127, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Saldaña, M.D.A. Subcritical water extraction of phenolic compounds from potato peel. Food Res. Int. 2011, 44, 2452–2458. [Google Scholar] [CrossRef]
- Tunchaiyaphum, S.; Eshtiaghi, M.N.; Yoswathana, N. Extraction of Bioactive Compounds from Mango Peels Using Green Technology. Int. J. Chem. Eng. Appl. 2013, 4, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Zhang, X.; He, L.; Yan, Q.; Yuan, F.; Gao, Y. Optimization of subcritical water extraction parameters of antioxidant polyphenols from sea buckthorn (Hippophaë rhamnoides L.) seed residue. J. Food Sci. Technol. 2015, 52, 1534–1542. [Google Scholar] [CrossRef] [Green Version]
- Nowak, A.; Czyzowska, A.; Efenberger, M.; Krala, L. Polyphenolic extracts of cherry (Prunus cerasus L.) and blackcurrant (Ribes nigrum L.) leaves as natural preservatives in meat products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef]
- Püssa, T.; Pallin, R.; Raudsepp, P.; Soidla, R.; Rei, M. Inhibition of lipid oxidation and dynamics of polyphenol content in mechanically deboned meat supplemented with sea buckthorn (Hippophae rhamnoides) berry residues. Food Chem. 2008, 107, 714–721. [Google Scholar] [CrossRef]
- Garrido, M.D.; Auqui, M.; Mart, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT Food Sci. Technol. 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Vaithiyanathan, S.; Naveena, B.M.; Muthukumar, M.; Girish, P.S.; Kondaiah, N. Effect of dipping in pomegranate (Punica granatum) fruit juice phenolic solution on the shelf life of chicken meat under refrigerated storage (4 °C). Meat Sci. 2011, 88, 409–414. [Google Scholar] [CrossRef]
- Huang, B.; He, J.; Ban, X.; Zeng, H.; Yao, X.; Wang, Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 2011, 87, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Latoch, A.; Stasiak, D.M. Effect of Mentha piperita on oxidative stability and sensory characteristics of cooked pork sausage. J. Food Process. Preserv. 2015, 39, 1566–1573. [Google Scholar] [CrossRef]





| Treatment | Content |
|---|---|
| 1 | Basic mass |
| 2 | Basic mass + 2% bilberry leaf |
| 3 | Basic mass + 1.6% sea buckthorn leaf |
| 4 | Basic mass + 0.2% bilberry leaf extract |
| 5 | Basic mass + 1.0% bilberry leaf extract |
| 6 | Basic mass + 0.2% sea buckthorn leaf extract |
| 7 | Basic mass + 1.0% sea buckthorn leaf extract |
| 8 | Basic mass + NaNO2 (0.1 g/kg mass) andascorbic acid (0.6 g/kg mass) |
| Treatment | Content |
|---|---|
| 1 | Basic marinade |
| 2 | Basic marinade + 4% bilberry leaf |
| 3 | Basic marinade + 4% sea buckthorn leaf |
| 4 | Basic marinade + 0.4% bilberry leaf extract |
| 5 | Basic marinade + 2% bilberry leaf extract |
| 6 | Basic marinade + 0.4% sea buckthorn leaf extract |
| 7 | Basic marinade + 2% sea buckthorn leaf extract |
| 8 | No marinade |
| Sample | Overall Preference | Color | Flavor |
|---|---|---|---|
| Bilberry leaf extract 0.2% A | 3.5 (1) | 3.4 (1) | 3.5 (1) |
| Bilberry leaf extract 0.2% B | 2.1 (5) | 2.5 (4) | 2.4 (5) |
| Bilberry leaf extract 1% | 1.8 (8) | 2.3 (5) | 1.8 (8) |
| Bilberry leaf 2% | 1.7 (9) | 1.5 (7) | 1.6 (9) |
| Sea buckthorn leaf extract 0.2% | 1.9 (7) | 2.0 (6) | 2.4 (5) |
| Sea buckthorn leaf extract 1% | 2.1 (5) | 0.8 (9) | 2.2 (7) |
| Sea buckthorn leaf 1.6% | 3.0 (3) | 2.7 (3) | 2.7 (3) |
| Basic mass | 2.3 (4) | 1.5 (7) | 2.5 (4) |
| Commercial mass | 3.2 (2) | 3.4 (1) | 3.5 (1) |
| Sample | Overall Preference | Color | Flavor |
|---|---|---|---|
| Bilberry leaf 4% | 3.0 1 (1) | 3.3 (3) | 2.8 (3) |
| Seabuckthorn leaf 4% | 3.0 1 (1) | 3.5 (1) | 3.3 (1) |
| Bilberry leaf extract 0.4% | 3.0 1 (1) | 2.5 (5) | 3.1 (2) |
| Bilberry leaf extract 2% | 2.3 (7) | 3.5 (1) | 2.5 (4) |
| Sea buckthorn leaf extract 0.4% | 2.5 (6) | 2.5 (5) | 1.9 (6) |
| Sea buckthorn leaf extract 2% | 2.6 (5) | 2.7 (4) | 1.8 (7) |
| Basic marinade | 3.0 1 (1) | 2.3 (7) | 2.0 (5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mäkinen, S.; Hellström, J.; Mäki, M.; Korpinen, R.; Mattila, P.H. Bilberry and Sea Buckthorn Leaves and Their Subcritical Water Extracts Prevent Lipid Oxidation in Meat Products. Foods 2020, 9, 265. https://doi.org/10.3390/foods9030265
Mäkinen S, Hellström J, Mäki M, Korpinen R, Mattila PH. Bilberry and Sea Buckthorn Leaves and Their Subcritical Water Extracts Prevent Lipid Oxidation in Meat Products. Foods. 2020; 9(3):265. https://doi.org/10.3390/foods9030265
Chicago/Turabian StyleMäkinen, Sari, Jarkko Hellström, Maarit Mäki, Risto Korpinen, and Pirjo H. Mattila. 2020. "Bilberry and Sea Buckthorn Leaves and Their Subcritical Water Extracts Prevent Lipid Oxidation in Meat Products" Foods 9, no. 3: 265. https://doi.org/10.3390/foods9030265