Autochthonous Saccharomyces cerevisiae Starter Cultures Enhance Polyphenols Content, Antioxidant Activity, and Anti-Inflammatory Response of Apulian Red Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
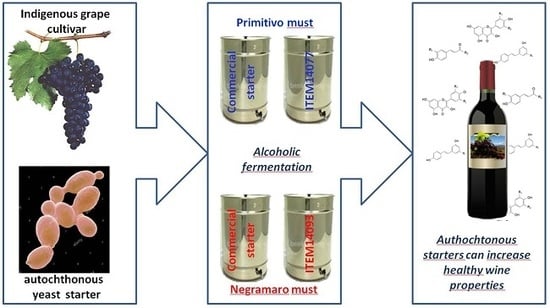
2.2. Wine Production
2.3. Red Wine Polyphenol Extraction and HPLC Analysis
2.4. Total Polyphenols Content
2.5. TEAC Antioxidant Capacity Determination
2.6. Cell Culture and Treatment
2.7. Cytotoxicity Assays
2.8. Endothelium-Monocyte Adhesion Assay
2.9. Detection of Endothelial Cell Surface Expression of VCAM-1
2.10. Statistical Analysis
3. Results
3.1. Industrial Vinification and Comparison of Chemical Parameters of Negroamaro and Primitivo Wines Obtained with Commercial or Autochthonous Yeast
3.2. Comparison of Polyphenols in Negroamaro and Primitivo Wine Extracts Obtained With Commercial or Autochthonous Yeast
3.3. Comparison of Total Polyphenols and Antioxidant Activity of Negroamaro and Primitivo Wines Obtained with Commercial or Autochthonous Yeast
3.4. Comparison of Vascular Anti-Inflammatory Response of Polyphenol Extracts from Negramaro and Primitivo Wines Obtained with Commercial or Autochthonous Yeast
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paixao, N.; Pereira, V.; Marques, J.C.; Camara, J.S. Quantification of polyphenols with potential antioxidant properties in wines using reverse phase HPLC. J. Sep. Sci. 2008, 31, 2189–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattivi, F.; Zulian, C.; Nicolini, G.; Valenti, L. Wine, biodiversity, technology, and antioxidants. Ann. N. Y. Acad. Sci. 2002, 957, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Sicari, V.; Pellicano, T.M.; Spizzirri, U.G.; Loizzo, M.R. The impact of cultivar on polyphenol and biogenic amine profiles in Calabrian red grapes during winemaking. Food Res. Int. 2017, 102, 303–312. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Grieco, F. Functional Properties of Grape and Wine Polyphenols. Plant Foods Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Kunsch, C.; Medford, R.M. Oxidative stress as a regulator of gene expression in the vasculature. Circ. Res. 1999, 85, 753–766. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J. Biol. Chem. 2006, 281, 37102–37110. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Pellegrino, M.; Storelli, C.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: differential role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression. Eur. J. Nutr. 2016, 55, 477–489. [Google Scholar] [CrossRef]
- Marzulli, G.; Magrone, T.; Kawaguchi, K.; Kumazawa, Y.; Jirillo, E. Fermented grape marc (FGM): immunomodulating properties and its potential exploitation in the treatment of neurodegenerative diseases. Curr. Pharmacol. Des. 2012, 18, 43–50. [Google Scholar] [CrossRef]
- Scarano, A.; Butelli, E.; De Santis, S.; Cavalcanti, E.; Hill, L.; De Angelis, M.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Santino, A. Combined Dietary Anthocyanins, Flavonols, and Stilbenoids Alleviate Inflammatory Bowel Disease Symptoms in Mice. Front. Nutr. 2018, 24, 4–75. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, G.; Ingrosso, I.; Paradiso, A.; De Gara, L.; Santino, A. Resveratrol Biosynthesis: Plant Metabolic Engineering for Nutritional Improvement of Food. Plant Foods Hum. Nutr. 2012, 67, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, V.; Fiore, C.; Maietti, A.; Tedeschi, P.; Romano, P. Influence of Saccharomyces cerevisiae strains on wine total antioxidant capacity evaluated by photo-chemiluminescence. World J. Microbiol. Biotechnol. 2007, 23, 581–586. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Carluccio, M.A.; Grieco, F. Wine polyphenols and health. In Bioactive Molecules in Food—Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K.G., Eds.; AG Springer Nature: Basel, Switzerland, 2019; pp. 1–21. [Google Scholar]
- Kostadinovic, S.; Wilkens, A.; Stefova, M.; Ivanova, V.; Vojnoski, B.; Mirhosseini, H.; Winterhalter, P. Stilbene levels and antioxidant activity of Vranec and Merlot wines from Macedonia: effect of variety and enological practices. Food Chem. 2012, 135, 3003–3009. [Google Scholar] [CrossRef] [PubMed]
- Carew, A.L.; Smith, P.; Close, D.C.; Curtin, C.; Dambergs, R.G. Yeast effects on Pinot noir wine phenolics, color, and tannin composition. J. Agric. Food Chem. 2013, 61, 9892–9898. [Google Scholar] [CrossRef]
- Carrascosa, A.V.; Bartolome, B.; Robredo, S.; Leon, A.; Cebollero, E.; Juega, M.; Nunez, Y.P.; Martinez, M.C.; Martinez-Rodriguez, A.J. Influence of locally-selected yeast on the chemical and sensorial properties of Albariño white wines. LWT Food Sci. Technol. 2012, 46, 319–325. [Google Scholar] [CrossRef]
- Caridi, A.; Sidari, R.; Solieri, L.; Cufari, A.; Giudici, P. Wine colour adsorption phenotype: inheritable quantitative trait loci of yeasts. J. Appl. Microbiol. 2007, 103, 735–742. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Sidari, R.; Postorino, S.; Caparello, A.; Caridi, A. Evolution during wine aging of colour and tannin differences induced by wine starters. Ann. Microbiol. 2007, 57, 197. [Google Scholar] [CrossRef]
- Tufariello, M.; Chiriatti, M.A.; Grieco, F.; Perrotta, C.; Capone, S.; Rampino, P.; Tristezza, M.; Mita, G.; Grieco, F. Influence of autochthonous Saccharomyces cerevisiae strains on volatile profile of Negroamaro wines. LWT Food Sci. Technol. 2014, 58, 35–48. [Google Scholar] [CrossRef]
- Tufariello, M.; Maiorano, G.; Rampino, P.; Spano, G.; Grieco, F.; Perrotta, C.; Capozzi, V.; Grieco, F. Selection of an autochthonous yeast starter culture for industrial production of Primitivo “Gioia del Colle” PDO/DOC in Apulia (Southern Italy). LWT Food Sci. Technol. 2019, 99, 188–196. [Google Scholar] [CrossRef]
- Tristezza, M.; Vetrano, C.; Bleve, G.; Grieco, F.; Tufariello, M.; Quarta, A.; Mita, G.; Spano, G.; Grieco, F. Autochthonous fermentation starters for the industrial production of Negroamaro wines. J. Ind. Microbiol. Biotechnol. 2012, 39, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Tristezza, M.; Gerardi, C.; Logrieco, A.; Grieco, F. An optimized protocol for the production of interdelta markers in Saccharomyces cerevisiae by using capillary electrophoresis. J. Microbiol. Methods 2009, 78, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Pellegrino, M.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Red Grape Skin Polyphenols Blunt Matrix Metalloproteinase-2 and -9 Activity and Expression in Cell Models of Vascular Inflammation: Protective Role in Degenerative and Inflammatory Diseases. Molecules 2016, 21, 1147. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fleet, G.H.; Heard, G.M. Yeast-Growth during Fermentation. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic: Lausanne, Switzerland, 1993; pp. 27–54. [Google Scholar]
- Gatto, P.; Vrhovsek, U.; Muth, J.; Segala, C.; Romualdi, C.; Fontana, P.; Pruefer, D.; Stefanini, M.; Moser, C.; Mattivi, F.; et al. Ripening and genotype control stilbene accumulation in healthy grapes. J. Agric. Food Chem. 2008, 56, 11773–11785. [Google Scholar] [CrossRef]
- Giordano, M.E.; Ingrosso, I.; Schettino, T.; Caricato, R.; Giovinazzo, G.; Lionetto, M.G. Intracellular Antioxidant Activity of Grape Skin Polyphenolic Extracts in Rat Superficial Colonocytes: In situ Detection by Confocal Fluorescence Microscopy. Front. Physiol. 2016, 7, 177. [Google Scholar] [CrossRef]
- Castillo-Munoz, N.; Gomez-Alonso, S.; Garcia-Romero, E.; Hermosin-Gutierrez, I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Duk, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: I. Analysis of remnant polyphenolic compounds in the resulting wines. J. Agric. Food Chem. 2005, 53, 5647–5653. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nogales, J.M.; Fernandez-Fernandez, E.; Gomez, M.; Vila-Crespo, J. Antioxidant properties of sparkling wines produced with beta-glucanases and commercial yeast preparations. J. Food Sci. 2012, 77, C1005–C1010. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, S.; Alexander, B.; Baranchuk, A. Wine and Cardiovascular Health: A Comprehensive Review. Circulation 2017, 136, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of yeast species and strains in wine flavor. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Fernandez, A.F.; Fraga, M.F. The effects of the dietary polyphenol resveratrol on human healthy aging and lifespan. Epigenetics 2011, 6, 870–874. [Google Scholar] [CrossRef] [PubMed]


| Wine | Alcohol | Sugars | AT | AV | Malic | Lactic | Tartaric | Citric | Glycerol | Methanol |
|---|---|---|---|---|---|---|---|---|---|---|
| P1A | 13.39 ± 0.003 | 2.06 ± 0.071 | 5.86 ± 0.026 | 0.39 ± 0.004 | 2.26 ± 0.027 | 0.26 ± 0.012 | 2.72 ± 0.048 | 0.23 ± 0.001 | 9.77 ± 0.02 | 0.06 ± 0.002 |
| P2A | 15.87 ± 0.011 | 2.47 ± 0.003 | 5.53 ± 0.044 | 0.36 ± 0.010 | 2.89 ± 0.029 | 0.19 ± 0.032 | 2.41 ± 0.058 | 0.22 ± 0.014 | 8.89 ± 0.034 | 0.08 ± 0.006 |
| P3A | 14.88 ± 0.104 | 2.37 ± 0.547 | 5.93 ± 0.117 | 0.43 ± 0.041 | 2.42 ± 0.363 | 0.29 ± 0.015 | 3.95 ± 0.042 | 0.28 ± 0.034 | 10.01 ± 0.171 | 0.05 ± 0.008 |
| N1A | 13.10 ± 0.009 | 2.36 ± 0.119 | 5.81 ± 0.020 | 0.40 ± 0.004 | 2.20 ± 0.019 | 0.27 ± 0.022 | 2.01 ± 0.009 | 0.24 ± 0.006 | 8.56 ± 0.045 | 0.05 ± 0.005 |
| N2A | 13.72 ± 0.018 | 1.42 ± 0.151 | 5.22 ± 0.007 | 0.42 ± 0.080 | 2.50 ± 0.003 | 0.32 ± 0.024 | 2.22 ± 0.014 | 0.12 ± 0.009 | 8.67 ± 0.075 | 0.03 ± 0.001 |
| N3A | 13.38 ± 0.034 | 0 | 5.29 ± .0.910 | 0.41 ± 0.040 | 2.10 ± 0.023 | 0.17 ± 0.014 | 2.48 ± 0.015 | 0.31 ± 0.012 | 8.41 ± 0.042 | 0.02 ± 0.002 |
| Groups | Compounds | P1C | P1A | P2C | P2A | P3C | P3A |
|---|---|---|---|---|---|---|---|
| mg/L | |||||||
| STILBENES | trans-Resveratrol | 3.7 ± 0.04 | 18.4 ± 0.31 * | 3.0 ± 0.07 | 23.0 ± 0.31 * | 0.9 ± 0.01 | 7.2 ± 0.06 * |
| trans-Piceid | 9.4 ± 0.01 | 41.8 ± 0.09 * | 9.2 ± 0.09 | 44.8 ± 0.27 * | 2.8 ± 0.095 | 11.2 ± 0.09 * | |
| total | 13.1 | 60.2 | 12.2 | 67.8 | 3.7 | 18.4 | |
| PHENOLIC ACIDS | Caftaric acid | 522.3 ± 5.9 | 1044.1 ± 3.1 * | 558.9 ± 4.87 | 1050 ± 6.9 * | 354.0 ± 3.72 | 637.0 ± 3.1 * |
| Caffeic acid | 30.0 ± 1.8 | 180.7 ± 1.0 * | 69.0 ± 0.7 | 180.5 ± 4.8 * | 69.7 ± 2.11 | 88.3 ± 2.11 * | |
| p-Coumaric acid | 6.4 ± 0.11 | 6.6 ± 0.7 | 4.32 ± 0.48 | 3.8 ± 0.02 | 3.8 ± 0.48 | 5,7 ± 2,8 * | |
| total | 558.7 | 1226.4 | 632.22 | 1233.8 | 427.57 | 731.0 | |
| FLAVONOLS | Myricetin | 3.3 ± 1.0 | 30.4 ± 1.4 * | 4.6 ± 0.45 | 25.2 ± 3.1 * | 7.0 ± 0.11 | 8.5 ± 0.95 * |
| Quercetin | 8.4 ± 0.7 | 46.9 ± 8.6 * | 9.9 ± 0.68 | 8.6 ± 1.0 | 7.6 ± 0.05 | 28.6 ± 0.8 * | |
| Kaempferol | 4.5 ± 0.1 | 11.5 ± 0.98 * | 5.4 ± 0.11 | 8.6 ± 0.7 * | 5.7 ± 0.02 | 7.9 ± 0.6 * | |
| total | 16.2 | 88.8 | 19.9 | 42.4 | 20.3 | 45.0 | |
| FLAVANOLS | Catechin | 8.8 + 0.2 | 8.9 ± 0.1 | 8.0 ± 0.1 | 8.5 ± 0.2 | 8.30 ± 0.5 | 8.9 ± 0.2 |
| Epicatechin | 9.4 ± 0.5 | 10.2 ± 0.5 | 8.07 ± 0.4 | 9.0 ± 0.4 | 9.5 + 0.51 | 9.2 + 1.1 | |
| total | 18.2 | 19.1 | 16.07 | 17.5 | 17.8 | 18.1 | |
| ** TP | mg GAEs/L | 928.8 ± 9.0 | 1366.6 ± 8.0 * | 1221.9 ± 7.6 | 1569.3 ± 7.6 * | 925.7 ± 6.8 | 1376.2 ± 8.0 * |
| § AA (TEAC) | mmol TE/100ml | 64.6 ± 1.1 | 96.4 ± 1.5 * | 77.3 ± 0.6 | 95.9 ± 1.7 * | 76.6 ± 2.1 | 96.03 ± 1.8 * |
| Groups | Compounds | N1C | N1A | N2C | N2A | N3C | N3A |
|---|---|---|---|---|---|---|---|
| mg/L | |||||||
| STILBENES | trans-Resveratrol | 4.8 ± 0.04 | 4.7 ± 0.05 | 3.2 ± 0.05 | 10.7 ± 0.7 * | 1.6 ± 0.04 | 7.8 ± 0.1 * |
| trans-Piceid | 14.03 ± 0.1 | 47.2 ± 1.0 * | 7.7 ± 0.1 | 28.9 ± 0.9 * | 5.5 ± 0.03 | 15.5 ± 0.08 * | |
| total | 18.83 | 51.9 | 10.9 | 39.6 | 7.1 | 23.3 | |
| PHENOLIC ACIDS | Caftaric acid | 574.5 ± 5.0 | 1373 ± 7.0 * | 484.3 ± 5.0 | 1633.9 ± 6.9 * | 293.5 ± 3.7 | 1267.2 ± 3.1 * |
| Caffeic acid | 96.9 ± 1.8 | 304.8 ± 1 * | 89.5 ± 0.7 | 334.8 ± 4.8 * | 53.5 ± 2.0 | 644.5 ± 2.1 * | |
| p-Coumaric acid | 6.4 ± 0.1 | 6.0 ± 0.4 | 4.3 ± 0.5 | 8.0 ± 0.02 * | 3.9 ± 0.98 | 5.7 ± 0,85 | |
| total | 677.8 | 1683.8 | 578.1 | 1976.7 | 350.9 | 1917.4 | |
| FLAVONOLS | Myricetin | 8 ± 0.1 | 7.8 ± 1.4 | 9.4 ± 0.45 | 14.1 ± 0.3 * | 0.37 ± 0.02 | 5.4 ± 0.9 * |
| Quercetin | 24.4 ± 0.7 | 23.3 ± 0.6 | 14.3 ± 0.7 | 65.9 ± 1 * | 1.3 ± 0.05 | 10 ± 0.8 * | |
| Kaempferol | 17.4 ± 0.1 | 26.8 ± 0.9 * | 3.3 ± 0.1 | 26.1 ± 0.7 * | 0.61 ± 0.02 | 1.70 ± 0.6 * | |
| total | 49.8 | 57.9 | 30.3 | 106.1 | 2.28 | 17.1 | |
| FLAVANOLS | Catechin | 8.0 ± 2.2 | 8.0 ± 0.1 | 8.2 ± 0.1 | 7.5 ± 0.2 | 7.30 ± 0.2 | 6.98 ± 0.5 |
| Epicatechin | 10.23 ± 2.12 | 12.2 ± 2.0 | 12.7 ± 2.2 | 15.0 ± 2.4 | 10.05 ± 2.0 | 11.21 ± 1.1 | |
| total | 18.23 | 20.2 | 20.9 | 22.5 | 17.35 | 18.19 | |
| ** TP | mg GAEs/L | 860.2 ± 9.0 | 1255.2 ± 8.0 * | 1118.5 ± 7.6 | 1245.5 ± 7.6 * | 971.2 ± 6.8 | 1277.3 ± 8.0 * |
| § AA (TEAC) | mmol TE/100ml | 62.9 ± 1.1 | 83 ± 1.5 * | 56.3 ± 0.6 | 83 ± 1.7 * | 50.3 ± 2.10 | 92.2 ± 1.8 * |
| NC | NA | PC | PA | |
|---|---|---|---|---|
| ** TP (mg GAEs/L) | 983.3 ± 9.5 | 1296.5 ± 10.3 * | 1025.5 ± 12.3 | 1102.1 ± 8.4 |
| § AA (mmol TE/L) | 565.3 ± 5.4 | 905.2 ± 7.8 * | 728.4 ± 6.5 | 961.3 ± 7.2 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grieco, F.; Carluccio, M.A.; Giovinazzo, G. Autochthonous Saccharomyces cerevisiae Starter Cultures Enhance Polyphenols Content, Antioxidant Activity, and Anti-Inflammatory Response of Apulian Red Wines. Foods 2019, 8, 453. https://doi.org/10.3390/foods8100453
Grieco F, Carluccio MA, Giovinazzo G. Autochthonous Saccharomyces cerevisiae Starter Cultures Enhance Polyphenols Content, Antioxidant Activity, and Anti-Inflammatory Response of Apulian Red Wines. Foods. 2019; 8(10):453. https://doi.org/10.3390/foods8100453
Chicago/Turabian StyleGrieco, Francesco, Maria Annunziata Carluccio, and Giovanna Giovinazzo. 2019. "Autochthonous Saccharomyces cerevisiae Starter Cultures Enhance Polyphenols Content, Antioxidant Activity, and Anti-Inflammatory Response of Apulian Red Wines" Foods 8, no. 10: 453. https://doi.org/10.3390/foods8100453