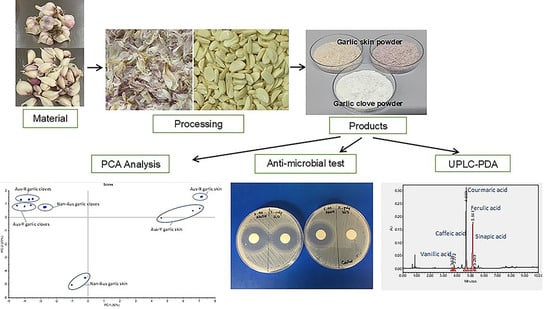
Phytochemical Characteristics and Antimicrobial Activity of Australian Grown Garlic (Allium Sativum L.) Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Analysis
2.3. Analysis of Polyphenols and Organosulfur Compounds
2.3.1. Extraction of Free Compounds
2.3.2. Extraction of Bound Phenolic Compounds
2.4. Total Phenolic Content and Antioxidant Capacity
2.5. Quantification of Polyphenols and Organosulfur Compounds
2.5.1. Phenolic Acids
2.5.2. Anthocyanins
2.5.3. Organosulfur Compounds
2.5.4. Identification of Polyphenols and Organosulfur Compounds
2.6. Antimicrobial Screening Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proximate
3.2. Total Phenolic Content (TPC)
3.3. Bioactive Compounds
3.3.1. Phenolic Acids and Anthocyanins
3.3.2. Organosulfur Compounds
3.4. ORAC Assay
3.5. Antimicrobial Activity
3.6. Multivariate Data Analysis
| 1. TPC-F | 10. L-Alliin | 19. Sinapic acid_B | 28. L. monocytogenes_W |
| 2. ORAC | 11. Allin isomer | 20. Vanillic acid_B | 29. P. aeruginosa_W |
| 3. Vanillic acid_F | 12. Methiin | 21. Total Phenolic acids_B | 30. C. albicans_W |
| 4. Caffeic acid_F | 13. Total organosulfur compounds | 22. Total Anthocyanins | 31. R. mucilaginosa_W |
| 5. p-Coumaric acid_F | 14. Total Phenolic acids_F | 23. S. aureus_W | 32. B. cereus_MeOH |
| 6. Ferulic acid- F | 15. TPC_B | 24. E. coli_W | 33. L. monocytogenes_MeOH |
| 7. Cyanidin-3-(6’-malonyl)-glucoside | 16. Caffeic acid_B | 25. S. aureus_MeOH | 34. P. aeruginosa_MeOH |
| 8. Cyanidin-based compound | 17. p-Coumaric acid_B | 26. E. coli_MEOH | 35. C. albicans_MeOH |
| 9. Pelargonidin-based compound | 18. Ferulic acid_B | 27. B. cereus_W | 36. R. mucilaginosa_MeOH |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Chatterjee, L.M.; Carlson, J.J. The effect of a garlic preparation on plasma lipid levels in moderately hypercholesterolemic adults. Atherosclerosis 2001, 154, 213–220. [Google Scholar] [CrossRef]
- Lawson, L.D.; Ransom, D.K.; Hughes, B.G. Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb. Res. 1992, 65, 141–156. [Google Scholar] [CrossRef]
- Hussain, S.P.; Jannu, L.N.; Rao, A.R. Chemopreventive action of garlic on methylcholanthrene-induced carcinogenesis in the uterine cervix of mice. Cancer Lett. 1990, 49, 175–180. [Google Scholar] [CrossRef]
- Tagoe, D.N.A.; Nyarko, H.D.; Akpaka, R. A comparison of Antifungal Properties of Onion (Allium cepa), Ginger (Zingiber officinale) and Garlic (Allium sativum) against Aspergillus flavus, Aspergillus niger and Cladosporium herbarum. Res. J. Med. Plants 2011, 5, 281–287. [Google Scholar] [CrossRef]
- Korukluoglu, R.I.A.M. Control of Aspergillus niger with garlic, onion and leek extracts. Afr. J. Biotechnol. 2007, 6, 384–387. [Google Scholar]
- Shrestha, D.K.; Sapkota, H.; Baidya, P.; Basnet, S. Antioxidant and Antibacterial Activities of Allium sativum and Allium Cepa. Bull. Pharm. Res. 2016, 6, 50–55. [Google Scholar] [CrossRef]
- FAO. FAO Statistic PocketBook: 2015; Food & Agriculture Organization: Rome, Italy, 2016. [Google Scholar]
- Lawson, L.D.; Hughes, B.G. Characterization of the Formation of Allicin and Other Thiosulfinates from Garlic. Planta Med. 1992, 58, 345–350. [Google Scholar] [CrossRef]
- Lawson, L.D.; Gardner, C.D. Composition, stability, and bioavailability of garlic products used in a clinical trial. J. Agric. Food Chem. 2005, 53, 6254–6261. [Google Scholar] [CrossRef]
- Lanzotti, V.; Romano, A.; Lanzuise, S.; Bonanomi, G.; Scala, F. Antifungal saponins from bulbs of white onion, Allium cepa L. Phytochemistry 2012, 74, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Parvu, M.; Parvu, E.A.; Toiu, A. Chemical Constituents of Three Allium Species from Romania. Molecules 2013, 18, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Drzewiecki, J.; Cvikrová, M.; Martincová, O.; Katrich, E.; Trakhtenberg, S. Comparison of the Main Bioactive Compounds and Antioxidant Activities in Garlic and White and Red Onions after Treatment Protocols. J. Agric. Food Chem. 2008, 56, 4418–4426. [Google Scholar] [CrossRef] [PubMed]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Goran, A.; Igic, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Du, C.T.; Francis, F.J. Anthocyanins of Garlic (Allium sativum L.). J. Food Sci. 1975, 40, 1101–1102. [Google Scholar] [CrossRef]
- Andersen, T.F.A.O.M. Malonated anthocyanins of garlic Allium sativum L. Food Chem. 1997, 58, 215–217. [Google Scholar]
- Martins, N.; Petropoulos, S.; Ferreira, I.C.F.R. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of Garlic Cultivars for Polyphenolic Content and Antioxidant Properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef]
- Gamboa, J.; Soria, A.C.; Corzo-Martinez, M.; Villamiel, A.M.a.M. Effect of storage on quality of industrially dehydrated onion, garlic, potato and carrot. J. Food Nutr. Res. 2012, 51, 132–144. [Google Scholar]
- Ichikawa, M.; Ide, N.; Yoshida, J.; Yamaguchi, H.; Ono, K. Determination of Seven Organosulfur Compounds in Garlic by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2006, 54, 1535–1540. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Masuero, D.; Mattivi, F.; Vrhovsek, U. Overall dietary polyphenol intake in a bowl of strawberries: The influence of Fragaria spp. in nutritional studies. J. Funct. Foods 2015, 18, 1057–1069. [Google Scholar] [CrossRef]
- Fredericks, C.H.; Fanning, K.J.; Gidley, M.J.; Netzel, G.; Zabaras, D.; Herrington, M.; Netzel, M. High-anthocyanin strawberries through cultivar selection. J. Sci. Food Agric. 2013, 93, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Beato, V.M.; Orgaz, F.; Mansilla, F.; Montaño, A. Changes in Phenolic Compounds in Garlic (Allium sativum L.) Owing to the Cultivar and Location of Growth. Plant Foods Hum. Nutr. 2011, 66, 218–223. [Google Scholar] [CrossRef]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006; pp. 3–19. [Google Scholar]
- Cheung Chung, S.W.; Kwong, K.P.; Yau, J.C.W.; Wong, W.W.K. Dietary exposure to antimony, lead and mercury of secondary school students in Hong Kong. Food Addit. Contam. Part A 2008, 25, 831–840. [Google Scholar] [CrossRef]
- EFSA_Panel_on_Contaminants_in_the_Food_Chain_(CONTAM). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar] [CrossRef]
- European_Food_Safety_Authority. Safety of aluminium from dietary intake—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J. 2008, 754, 1–34. [Google Scholar] [CrossRef]
- Naheed, Z.; Cheng, Z.H.; Wu, C.N.; Wen, Y.B.; Ding, H.Y. Total polyphenols, total flavonoids, allicin and antioxidant capacities in garlic scape cultivars during controlled atmosphere storage. Postharvest Biol. Technol. 2017, 131, 39–45. [Google Scholar] [CrossRef]
- Nuutila, A.M.; Puupponen-Pimiä, R.; Aarni, M.; Oksman-Caldentey, K.-M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Toledano Medina, M.Á.; Pérez-Aparicio, J.; Moreno-Ortega, A.; Moreno-Rojas, R. Influence of Variety and Storage Time of Fresh Garlic on the Physicochemical and Antioxidant Properties of Black Garlic. Foods 2019, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Rybczyńska-Tkaczyk, K.; Gaweł-Bęben, K.; Świeca, M.; Karaś, M.; Jakubczyk, A.; Matysiak, M.; Binduga, U.E.; Gmiński, J. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Pol. J. Food Nutr. Sci. 2018, 68, 73–81. [Google Scholar] [CrossRef]
- Fossen, T.; Andersen, Ø.M.; ØVstedal, D.O.; Pedersen, A.T.; Raknes, Å. Characteristic Anthocyanin Pattern from Onions and other Allium spp. J. Food Sci. 1996, 61, 703–706. [Google Scholar] [CrossRef]
- Dufoo-Hurtado, M.D.; Zavala-Gutiérrez, K.G.; Cao, C.-M.; Cisneros-Zevallos, L.; Guevara-González, R.G.; Torres-Pacheco, I.; Vázquez-Barrios, M.E.; Rivera-Pastrana, D.M.; Mercado-Silva, E.M. Low-Temperature Conditioning of “Seed” Cloves Enhances the Expression of Phenolic Metabolism Related Genes and Anthocyanin Content in ‘Coreano’ Garlic (Allium sativum) during Plant Development. J. Agric. Food Chem. 2013, 61, 10439–10446. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Flores, M.I.; Romero-Gonzalez, R.; Vidal, J.L.M.; Frenich, A.G. Determination of Phenolic Compounds in Artichoke, Garlic and Spinach by Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Food Anal. Methods 2014, 7, 2095–2106. [Google Scholar] [CrossRef]
- Horníčková, J.; Kubec, R.; Cejpek, K.; Velíšek, J.; Ovesná, J.; Stavělíková, H. Profiles of S-alk(en)ylcysteine sulfoxides in various garlic genotypes. Czech J. Food Sci. 2010, 28, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.C.; Kakino, K.; Nogami, C.; Ohnuki, K.; Shimizu, K. An LC-MS/MS-SRM Method for Simultaneous Quantification of Four Representative Organosulfur Compounds in Garlic Products. Food Anal. Methods 2016, 9, 3378–3384. [Google Scholar] [CrossRef]
- Krest, I.; Glodek, J.; Keusgen, M. Cysteine sulfoxides and alliinase activity of some Allium species. J. Agric. Food Chem. 2000, 48, 3753–3760. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.B.; Jin, W.; Park, J.; Yoon, W.; Lee, Y.; Kim, S.; Lee, S.; Kim, S.; Lee, O.H.; et al. Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.). Nat. Prod. Res. 2017, 32, 1193–1197. [Google Scholar] [CrossRef]
- Yin, M.-C.; Cheng, W.-S. Antioxidant Activity of Several Allium Members. J. Agric. Food Chem. 1998, 46, 4097–4101. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.B.; Lee, S.; Park, J.; Shin, D.; Yoo, M. Profiling of organosulphur compounds using HPLC-PDA and GC/MS system and antioxidant activities in hooker chive (Allium hookeri). Nat. Prod. Res. 2016, 30, 2798–2804. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.Y. The antioxidant properties of garlic compounds: Allyl cysteine, alliin, allicin, and allyl disulfide. J. Med. Food 2006, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Rasul Suleria, H.A.; Sadiq Butt, M.; Muhammad Anjum, F.; Saeed, F.; Batool, R.; Nisar Ahmad, A. Aqueous garlic extract and its phytochemical profile; special reference to antioxidant status. Int. J. Food Sci. Nutr. 2012, 63, 431–439. [Google Scholar] [CrossRef] [PubMed]
- El-Hamidi, M.; El-Shami, S.M. Scavenging activity of different garlic extracts and garlic powder and their antioxidant effect on heated sunflower oil. Am. J. Food Technol. 2015, 10, 135–146. [Google Scholar] [CrossRef]
- Rees, L.P.; Minney, S.F.; Plummer, N.T.; Slater, J.H.; Skyrme, D.A. A quantitative assessment of the antimicrobial activity of garlic (Allium sativum). World J. Microbiol. Biotechnol. 1993, 9, 303–307. [Google Scholar] [CrossRef]
- Hughes, B.G.; Lawson, L.D. Antimicrobial effects of Allium sativum L. (garlic), Allium ampeloprasum L. (elephant garlic), and Allium cepa L. (onion), garlic compounds and commercial garlic supplement products. Phytother. Res. 1991, 5, 154–158. [Google Scholar] [CrossRef]





| Proximate Composition | Unit | Non-AustralianGarlic Clove | AustralianX GarlicClove | AustralianY GarlicClove | DRI * | |
|---|---|---|---|---|---|---|
| Energy | kJ/100 g | 1454 | 1477 | 1457 | ||
| Protein | g/100 g | 16.8 | 22.8 | 23.2 | ||
| Fat | Total fat | g/100 g | 9.1 | 8.8 | 8.2 | |
| Saturated fat | 2.0 | 1.9 | 1.8 | |||
| Monounsaturated fat | 0.4 | 0.6 | 0.7 | |||
| Polyunsaturated fat | 6.7 | 6.3 | 5.8 | |||
| Trans fat | <0.01 | <0.01 | <0.01 | |||
| Carbohydrate | Total carbohydrate | g/100 g | 31.6 | 28.4 | 28.3 | |
| Total sugar | 3.2 | 3.0 | 2.7 | |||
| Dietary fiber | Total dietary fiber | g/100 g | 36.9 | 34.9 | 34.7 | |
| Crude fiber | 0.9 | 0.6 | 0.2 | |||
| Minerals | Sodium (Na) | mg/100 g | 29 | 9.4 | 13 | 1.3 g AI [28] |
| Potassium (K) | 1,580 | 1,310 | 1,330 | 4.7 g AI [28] | ||
| Iron (Fe) | 2.9 | 2.4 | 1.7 | 8 mg RDA [28] | ||
| Calcium (Ca) | 47 | 34 | 36 | 1200 mg AI [28] | ||
| Magnesium (Mg) | 85 | 62 | 57 | 350 mg EAR [28] | ||
| Zinc (Zn) | 2.2 | 2.2 | 2.4 | 11 mg RDA [28] | ||
| Heavy metals | Mercury (Hg) Lead (Pb) Cadmium (Cd) Aluminum (Al) Chromium (Cr) | mg/kg | <0.01 <0.01 0.034 0.43 0.073 | <0.01 <0.01 0.023 0.52 0.083 | <0.01 <0.01 0.012 0.88 0.073 | 5 µg/kg BW/week UL [29] 25 µg/kg BW/week UL [29] 2.5 µg/kg BW/week UL [30] 1.0 mg/kg BW/week UL [31] 35 µg/day AI [29] |
| Moisture content | % | 1.6 | 1.5 | 2.0 | ||
| Ash | % | 3.9 | 3.5 | 3.5 | ||
| Tentative Identified Compound | Retention Time (min) | UHPLC-PDA ʎmax (nm) | [M–H]–m/z | Previous Reports |
|---|---|---|---|---|
| Phenolic acids | ||||
| Vanillic acid | 3.6 | 280 | 167.0338 | [14,27] |
| Caffeic acid | 3.8 | 280 | 179.0438 | [14,27,38] |
| p-Coumaric acid | 4.6 | 280 | 163.0401 | [13] |
| Ferulic acid | 5.1 | 280 | 193.0495 | [13,27,38] |
| Sinapic acid | 5.3 | 280 | 223.0603 | [13,14,27] |
| Anthocyanins | [M–H]+ and fragment MS2 | |||
| Cyanidin-3-(6’-malonyl)-glucoside) | 3.5 | 520 | 535.1024, 287.0550 | [17,36,37] |
| Cyanidin-based compound | 5.2 | 520 | 862.2545, 538.1505, 287.0550 | [16,37] |
| Pelargonidin-based compound | 5.4 | 520 | 447.3906, 271.2058 |
| Phenolic Compounds | Non-Australian Garlic | Australian X Garlic | Australian Y Garlic | |||
|---|---|---|---|---|---|---|
| Clove | Skin | Clove | Skin | Clove | Skin | |
| Free phenolic acids (mg/100 g DW) | ||||||
| Vanillic acid | - | 0.6 ± 0.06 a | - | 0.4 ± 0.02 b | - | 0.5 ± 0.02 b |
| Caffeic acid | 0.1 ± 0.01 c (*) | 0.6 ± 0.17 b | 0.11 ± 0.04 c | 0.9 ± 0.02 ab | 0.13 ± 0.02 c | 0.9 ± 0.02 a |
| p-Coumaric acid | - | - | - | 8.0 ± 0.3 a | - | 5.7 ± 0.4 b |
| Ferulic acid | - | 1.7 ± 0.04 c | - | 15.2 ± 0.8 b | - | 30.5 ± 1.2 a |
| Sum | 0.1 ± 0.01 d | 2.9 ± 0.2 c | 0.11 ± 0.04 d | 24.4 ± 0.9 b | 0.13 ± 0.02 d | 37.5 ± 1.5 a |
| Bound phenolic acids (mg/100 g DW) | ||||||
| Vanillic acid | 0.02 ± 0.01 c | 1.0 ± 0.06 a | - | 0.2 ± 0.01 b | - | 0.3 ± 0.03 b |
| Caffeic acid | 0.04 ± 0.02 d | 0.5 ± 0.04 c | 0.02 ± 0.001 d | 0.9 ± 0.04 b | 0.02 ± 0.001 d | 1.3 ± 0.05 a |
| p-Coumaric acid | 0.05 ± 0.001 d | 4.0 ± 0.28 c | 0.2 ± 0.01 d | 16.1 ± 0.3 b | 0.14 ± 0.02 d | 33.2 ± 0.5 a |
| Ferulic acid | 0.07 ± 0.001 d | 2.5 ± 0.05 c | 0.1 ± 0.02 d | 21.7 ± 0.6 b | 0.11 ± 0.02 d | 37.4 ± 0.5 a |
| Sinapic acid | 0.4 ± 0.01 d | 3.0 ± 0.34 c | - | 6.9 ± 0.3 a | - | 3.9 ± 0.2 b |
| Sum | 0.6 ± 0.04 d | 11.0 ± 0.7 c | 0.3 ± 0.03 d | 45.9 ± 0.6 b | 0.4 ± 0.01 d | 76.1 ± 1.1 a |
| Anthocyanins (mg Cya-3-glc equivalents/100 g DW) | ||||||
| Cyanidin-3-(6’-malonyl)-glucoside | - | - | - | 0.2 ± 0.02 a | - | 0.2 ± 0.01 a |
| Cyanidin-based compound | - | - | - | 0.03 ± 0.001 a | - | 0.02 ± 0.001 a |
| Pelargonidin-based compound | - | - | - | 0.1 ± 0.01 a | - | 0.09 ± 0.01 a |
| Sum | 0.3 ± 0.02 a | 0.3 ± 0.02 a | ||||
| Tentative Identified Compound | Retention Time (min) | UPLC-PDA ʎmax (nm) | [M–H]–m/z | Previous Reports |
|---|---|---|---|---|
| L-alliin Alliin isomer Methiin | 5.8 7.6 9.0 | 210 210 210 | 178.0532, 88.0398 178.0532, 88.0398 152.0375 | [21,40,41] [21] [21,41] |
| Microorganism | Negative Control (20% Ethanol) | Non-Australian Garlic | Australian X Garlic | Australian Y Garlic | |||
|---|---|---|---|---|---|---|---|
| Clove | Skin | Clove | Skin | Clove | Skin | ||
| Water extraction | |||||||
| B. cereus | - | - | - | 21.7 ± 1.2 a * | - | 21.9 ± 1.3 a | - |
| L. monocytogenes | - | 32.9 ± 0.5 a | - | 33.9 ± 1.2 a | - | 33.5 ± 1.5 a | - |
| P. aeruginosa | - | - | - | - | - | - | - |
| C. albicans | - | 23.9 ± 0.7 a | - | 23.5 ± 3.7 a | 16 ± 0.5 b | 24.5 ± 0.8 a | 22.7 ± 1.3 a |
| R. mucilaginosa | - | 27.2 ± 1.2 ab | - | 27.1 ± 0.4 b | 17.6 ± 2.4 d | 30.7 ± 0.8 a | 23.4 ± 0.8 c |
| S.aureus | - | 18.9 ± 0.5 c | - | 19.1 ± 1.1 c | 22 ± 0.6 b | 24.8 ± 0.6 a | - |
| E. Coli | - | 16.9 ± 0.1 a | - | 15.8 ± 0.7 a | 16.8 ± 0.6 a | Possibly partial inhibition | - |
| Methanolic extraction | |||||||
| B. cereus | - | 22.7 ± 0.6 c | - | 30 ± 1.8 a | - | 25.6 ± 1 b | 13.3 ± 0.5 d |
| L. monocytogenes | - | 24.9 ± 0.7 a | - | 25 ± 1.5 a | 19.2 ± 0.4 b | 20.6 ± 0.8 b | 19.2 ± 0.7 b |
| P. aeruginosa | - | - | - | 13.5 ± 0.4 a | - | 14.0 ± 0.5 a | - |
| C. albicans | - | 38.3 ± 1.6 ab | - | 40.9 ± 1.5 a | 21.3 ± 1.1 c | 37.1 ± 0.7 b | 23.9 ± 1.2 c |
| R. mucilaginosa | - | 37.3 ± 0.9 a | - | 37 ± 0.4 a | 24.0 ± 2.2 c | 32.0 ± 1.1 b | 27.3 ± 0.9 c |
| S. aureus | - | 27.3 ± 4.7 ab | - | 34.3 ± 2.1 a | 20.5 ± 0.3 bc | 30.0 ± 3.6 a | 19.4 ± 0.5 c |
| E. Coli | - | 19.8 ± 0.1 b | - | 23.9 ± 0.6 a | - | 19.6 ± 0.5 b | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, A.D.T.; Netzel, G.; Chhim, P.; Netzel, M.E.; Sultanbawa, Y. Phytochemical Characteristics and Antimicrobial Activity of Australian Grown Garlic (Allium Sativum L.) Cultivars. Foods 2019, 8, 358. https://doi.org/10.3390/foods8090358
Phan ADT, Netzel G, Chhim P, Netzel ME, Sultanbawa Y. Phytochemical Characteristics and Antimicrobial Activity of Australian Grown Garlic (Allium Sativum L.) Cultivars. Foods. 2019; 8(9):358. https://doi.org/10.3390/foods8090358
Chicago/Turabian StylePhan, Anh Dao Thi, Gabriele Netzel, Panhchapor Chhim, Michael E. Netzel, and Yasmina Sultanbawa. 2019. "Phytochemical Characteristics and Antimicrobial Activity of Australian Grown Garlic (Allium Sativum L.) Cultivars" Foods 8, no. 9: 358. https://doi.org/10.3390/foods8090358