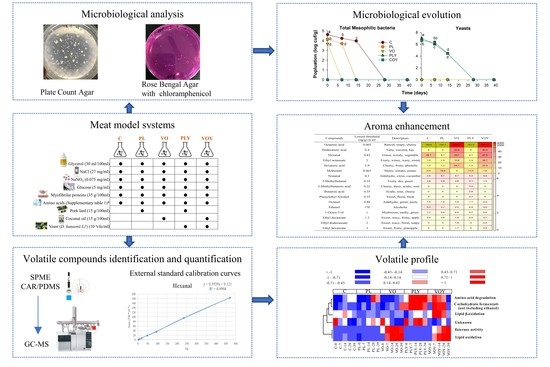
Short-Term Changes in Aroma-Related Volatiles in Meat Model: Effect of Fat and D. hansenii Inoculation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Preparation of the Yeast Starter
2.3. Preparation of the Meat Model Systems
2.4. Microbial Analysis
2.5. Analysis of Volatile Compounds
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Belloch, C.; Flores, M. The Maillard Reaction as Source of Meat Flavor Compounds in Dry Cured Meat Model Systems under Mild Temperature Conditions. Molecules 2021, 26, 223. [Google Scholar] [CrossRef]
- Perea-Sanz, L.; Peris, D.; Belloch, C.; Flores, M. Debaryomyces hansenii Metabolism of Sulfur Amino Acids As Precursors of Volatile Sulfur Compounds of Interest in Meat Products. J. Agric. Food Chem. 2019, 67, 9335–9343. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Zamora, R. Amino Acid Degradations Produced by Lipid Oxidation Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1242–1252. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, F.J.; Zamora, R. Formation of phenylacetic acid and benzaldehyde by degradation of phenylalanine in the presence of lipid hydroperoxides: New routes in the amino acid degradation pathways initiated by lipid oxidation products. Food Chem. X 2019, 2, 100037. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Durá, M.A.; Marco, A.; Toldrá, F. Effect of Debaryomyces spp. on aroma formation and sensory quality of dry-fermented sausages. Meat Sci. 2004, 68, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Gómez, M.; Purriños, L.; Fonseca, S. Effect of commercial starter cultures on volatile compound profile and sensory characteristics of dry-cured foal sausage. J. Sci. Food Agric. 2016, 96, 1194–1201. [Google Scholar] [CrossRef]
- Corral, S.; Salvador, A.; Belloch, C.; Flores, M. Improvement the aroma of reduced fat and salt fermented sausages by Debaromyces hansenii inoculation. Food Control 2015, 47, 526–535. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Juan, M.; Flores, M.; Toldrá, F. Effect of pork meat proteins on the binding of volatile compounds. Food Chem. 2008, 108, 1226–1233. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.L.; Salvador, A.; Flores, M. Sensory acceptability of slow fermented sausages based on fat content and ripening time. Meat Sci. 2010, 86, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Corral, S.; Leitner, E.; Siegmund, B.; Flores, M. Determination of sulfur and nitrogen compounds during the processing of dry fermented sausages and their relation to amino acid generation. Food Chem. 2016, 190, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Belloch, C.; Neef, A.; Salafia, C.; López-Diez, J.J.; Flores, M. Microbiota and volatilome of dry-cured pork loins manufactured with paprika and reduced concentration of nitrite and nitrate. Food Res. Int. 2021, 149, 110691. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Wieczorek, M.N. Commentary: “Quantitative” vs quantitative Headspace Solid-Phase Microextraction (HS-SPME) in food volatile and flavor compounds analysis. J. Food Compos. Anal. 2023, 115, 104955. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011; pp. 70–452. [Google Scholar]
- Belloch, C.; Perea-Sanz, L.; Gamero, A.; Flores, M. Selection of Debaryomyces hansenii isolates as starters in meat products based on phenotypic virulence factors, tolerance to abiotic stress conditions and aroma generation. J. Appl. Microbiol. 2022, 133, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Perea-Sanz, L.; Salvador, A.; Belloch, C.; Flores, M. Understanding the impact of nitrogen and sulfur precursors on the aroma of dry fermented sausages. Meat Sci. 2022, 192, 108896. [Google Scholar] [CrossRef] [PubMed]
- Perea-Sanz, L.; López-Díez, J.J.; Belloch, C.; Flores, M. Counteracting the effect of reducing nitrate/nitrite levels on dry fermented sausage aroma by Debaryomyces hansenii inoculation. Meat Sci. 2020, 164, 108103. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and Analysis of Volatile and Odor Compounds in Meat—A Review. Molecules 2022, 27, 6703. [Google Scholar] [CrossRef] [PubMed]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [Green Version]
- Pinho, O.; Ferreira, I.M.P.L.V.O.; Ferreira, M.A. Solid-phase microextraction in combination with GC/MS for quantification of the major volatile free fatty acids in ewe cheese. Anal. Chem. 2002, 74, 5199–5204. [Google Scholar] [CrossRef]
- Daza, A.; Menoyo, D.; Olivares, A.; Cordero, G.; López-Bote, C.J. Effect of Iberian pig feeding system on tissue fatty-acid composition and backfat rheological properties. J. Anim. Feed Sci. 2007, 16, 408–419. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-García, L.; Rivera-Jiménez, S.; Belloch, C.; Flores, M. Generation of aroma compounds in a fermented sausage meat model system by Debaryomyces hansenii strains. Food Chem. 2014, 151, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Mi, R.; Chen, X.; Zhu, Q.; Xiong, S.; Qi, B.; Wang, S. Evaluation and selection of yeasts as potential aroma enhancers for the production of dry-cured ham. Food Sci. Hum. Wellness 2022, 12, 324–335. [Google Scholar] [CrossRef]
- Olesen, P.T.; Stahnke, L.H. The influence of Debaryomyces hansenii and Candida utilis on the aroma formation in garlic spiced fermented sausages and model minces. Meat Sci. 2000, 56, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, Z.; Meng, Q.; Li, D.; Ding, Z. Key aroma compounds and metabolic profiling of Debaryomyces hansenii L1-1-fermented Flos Sophorae. J. Food Biochem. 2021, 45, e13711. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. The Maillard reaction and lipid oxidation. Lipid Technol. 2011, 23, 59–62. [Google Scholar] [CrossRef]
- Smit, B.A.; Engels, W.J.M.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, L.M.; Gori, K.; Petersen, M.A.; Jespersen, L.; Arneborg, N. Flavour compound production by Yarrowia lipolytica, Saccharomyces cerevisiae and Debaryomyces hansenii in a cheese-surface model. Int. Dairy J. 2011, 21, 970–978. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, P. Reactive Carbonyl Species Derived from Omega-3 and Omega-6 Fatty Acids. J. Agric. Food Chem. 2015, 63, 6293–6296. [Google Scholar] [CrossRef] [Green Version]
- Lapadatescu, C.; Giniès, C.; Le Quéré, J.L.; Bonnarme, P. Novel scheme for biosynthesis of aryl metabolites from L-phenylalanine in the fungus Bjerkandera adusta. Appl. Environ. Microbiol. 2000, 66, 1517–1522. [Google Scholar] [CrossRef] [Green Version]
- Baldovini, N.; Chaintreau, A. Identification of key odorants in complex mixtures occurring in nature. Nat. Prod. Rep. 2020, 37, 1589–1626. [Google Scholar] [CrossRef] [PubMed]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef] [PubMed]




| Meat Model Systems 1 | C | PL | VO | PLY | VOY |
|---|---|---|---|---|---|
| pH | 5 | 5 | 5 | 5 | 5 |
| aw 2 | 0.895 | 0.895 | 0.895 | 0.895 | 0.895 |
| NaCl (%) | 2.7 | 2.7 | 2.7 | 2.7 | 2.7 |
| NaNO3 (%) | 0.0075 | 0.0075 | 0.0075 | 0.0075 | 0.0075 |
| Glucose (%) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Amino acids 3 | + | + | + | + | + |
| Myofibrillar proteins (%) | 35 | 35 | 35 | 35 | 35 |
| Pork lard (%) | 15 | 15 | |||
| Coconut oil (%) | 15 | 15 | |||
| Yeast (D. hansenii L1) (CFU ml−1) | 106 | 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Belloch, C.; Flores, M. Short-Term Changes in Aroma-Related Volatiles in Meat Model: Effect of Fat and D. hansenii Inoculation. Foods 2023, 12, 2429. https://doi.org/10.3390/foods12122429
Li L, Belloch C, Flores M. Short-Term Changes in Aroma-Related Volatiles in Meat Model: Effect of Fat and D. hansenii Inoculation. Foods. 2023; 12(12):2429. https://doi.org/10.3390/foods12122429
Chicago/Turabian StyleLi, Lei, Carmela Belloch, and Mónica Flores. 2023. "Short-Term Changes in Aroma-Related Volatiles in Meat Model: Effect of Fat and D. hansenii Inoculation" Foods 12, no. 12: 2429. https://doi.org/10.3390/foods12122429